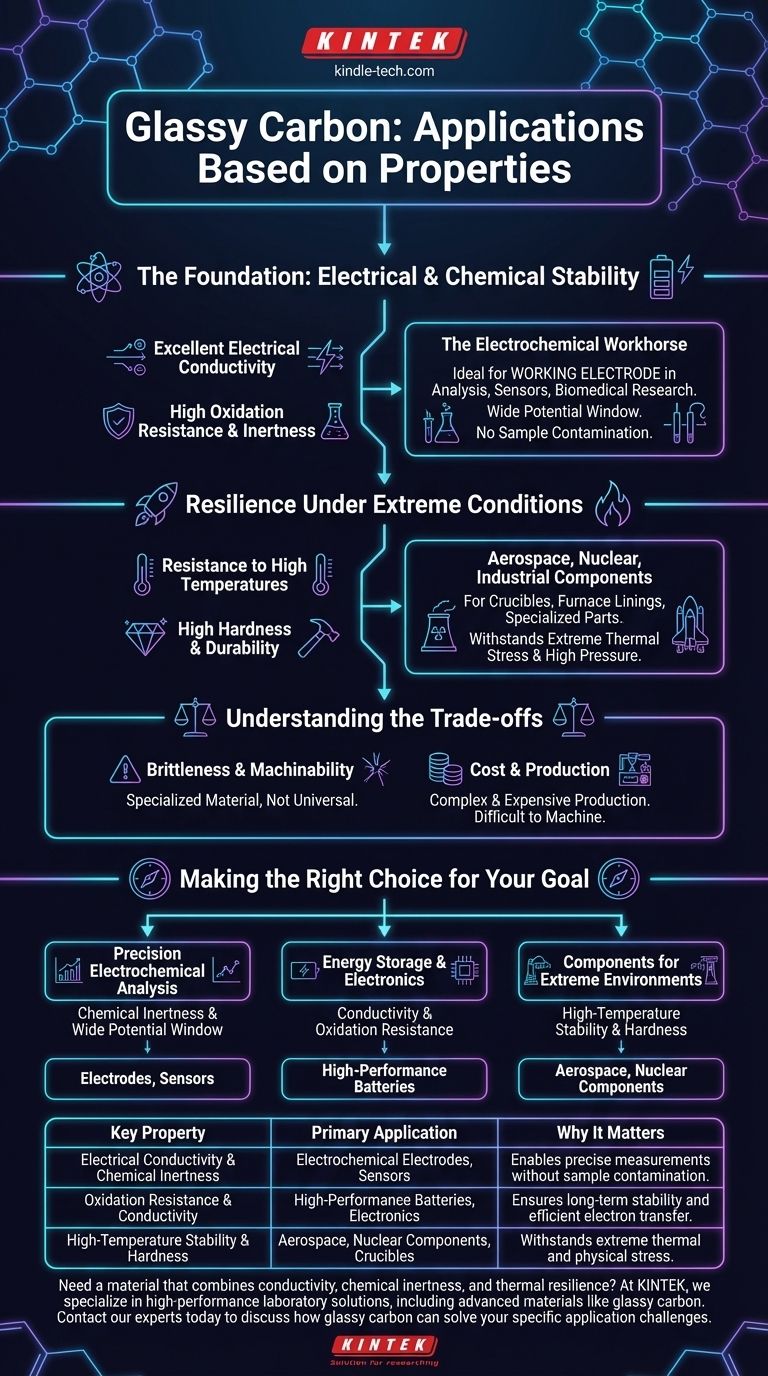
At its core, glassy carbon is a high-performance material primarily used in three key areas: advanced electrochemistry, high-performance electronics and batteries, and components for extreme environments like aerospace and nuclear energy. Its applications are a direct result of its unique combination of properties, which resemble a glass, a ceramic, and a graphite all at once.
Glassy carbon's value lies not in a single trait, but in its rare blend of electrical conductivity, extreme chemical inertness, and high-temperature resilience. Understanding which property drives which application is the key to leveraging this specialized material effectively.

The Foundation: Electrical and Chemical Stability
The most common applications for glassy carbon stem from its exceptional performance as an electrical conductor that does not chemically interfere with its environment.
Excellent Electrical Conductivity
Glassy carbon conducts electricity well, a trait it shares with graphite. This property is fundamental to its use in high-performance batteries and other electronic devices where efficient electron transfer is critical.
High Oxidation Resistance and Inertness
Unlike many conductive materials, glassy carbon is highly resistant to oxidation and is chemically inert across a wide range of conditions. It does not easily react with acids, bases, or other corrosive agents.
The Electrochemical Workhorse
This combination of conductivity and inertness makes glassy carbon an ideal material for electrodes. It is widely used as the working electrode in electrochemical analysis, sensors, and biomedical research because it facilitates measurements without contaminating or reacting with the sample. Its wide potential window means it remains stable over a broad range of voltages.
Resilience Under Extreme Conditions
For more demanding physical applications, glassy carbon's structural and thermal properties become the main attraction. It performs where many conventional materials would fail.
Resistance to High Temperatures
Glassy carbon maintains its structural integrity at very high temperatures. This makes it a valuable material for components in fields like aerospace and nuclear energy, which involve extreme thermal stress.
High Hardness and Durability
Along with thermal stability, the material exhibits high hardness. This durability allows it to withstand the high-pressure and physically demanding environments characteristic of its use in crucibles, furnace linings, and specialized industrial components.
Understanding the Trade-offs
While powerful, glassy carbon is a specialized material with inherent limitations that define its use cases. It is not a universal solution.
Brittleness and Machinability
Like many hard, ceramic-like materials, glassy carbon is brittle. It can fracture under sharp impact and is difficult to machine into complex shapes compared to metals, which influences component design.
Cost and Production
Glassy carbon is produced through a complex, slow process of pyrolyzing (heating) a polymer precursor. This makes it significantly more expensive than standard graphite or other common conductors, restricting its use to applications where its unique benefits justify the cost.
Making the Right Choice for Your Goal
To determine if glassy carbon is the correct choice, you must align its specific strengths with your primary objective.
- If your primary focus is precision electrochemical analysis: Its chemical inertness and wide potential window make it the superior choice for sensitive and accurate measurements.
- If your primary focus is energy storage and electronics: Its combination of electrical conductivity and resistance to oxidation ensures long-term stability and performance in batteries.
- If your primary focus is components for extreme environments: Its high-temperature stability and hardness are the critical properties for reliability in aerospace or nuclear applications.
Ultimately, glassy carbon serves as a premier problem-solving material for challenges that demand a rare intersection of electrical, chemical, and physical resilience.
Summary Table:
| Key Property | Primary Application | Why It Matters |
|---|---|---|
| Electrical Conductivity & Chemical Inertness | Electrochemical Electrodes, Sensors | Enables precise measurements without sample contamination. |
| Oxidation Resistance & Conductivity | High-Performance Batteries, Electronics | Ensures long-term stability and efficient electron transfer. |
| High-Temperature Stability & Hardness | Aerospace, Nuclear Components, Crucibles | Withstands extreme thermal and physical stress. |
Need a material that combines conductivity, chemical inertness, and thermal resilience?
At KINTEK, we specialize in providing high-performance laboratory equipment and consumables, including solutions utilizing advanced materials like glassy carbon. Whether you are developing sensitive sensors, high-capacity batteries, or components for extreme environments, our expertise can help you select the right materials for superior performance and reliability.
Contact our experts today to discuss how glassy carbon can solve your specific application challenges.
Visual Guide
Related Products
- Conductive Carbon Cloth Carbon Paper Carbon Felt for Electrodes and Batteries
- Electrode Polishing Material for Electrochemical Experiments
- High Purity Zinc Foil for Battery Lab Applications
- Aluminum Foil Current Collector for Lithium Battery
- Button Battery Storage Box for Battery Lab
People Also Ask
- What are 3 products that carbon nanotubes can be used in? Enhancing Batteries, Tires, and Composites
- What are the three types of coating? A Guide to Architectural, Industrial, and Special Purpose
- What is the ideal operating environment for a glassy carbon sheet? Ensure Optimal Performance and Longevity
- How should carbon cloth used for high-temperature electrolysis be handled after operation? Prevent Irreversible Oxidative Damage
- What applications is carbon felt suitable for? Ideal for High-Performance Electrochemical Systems



















