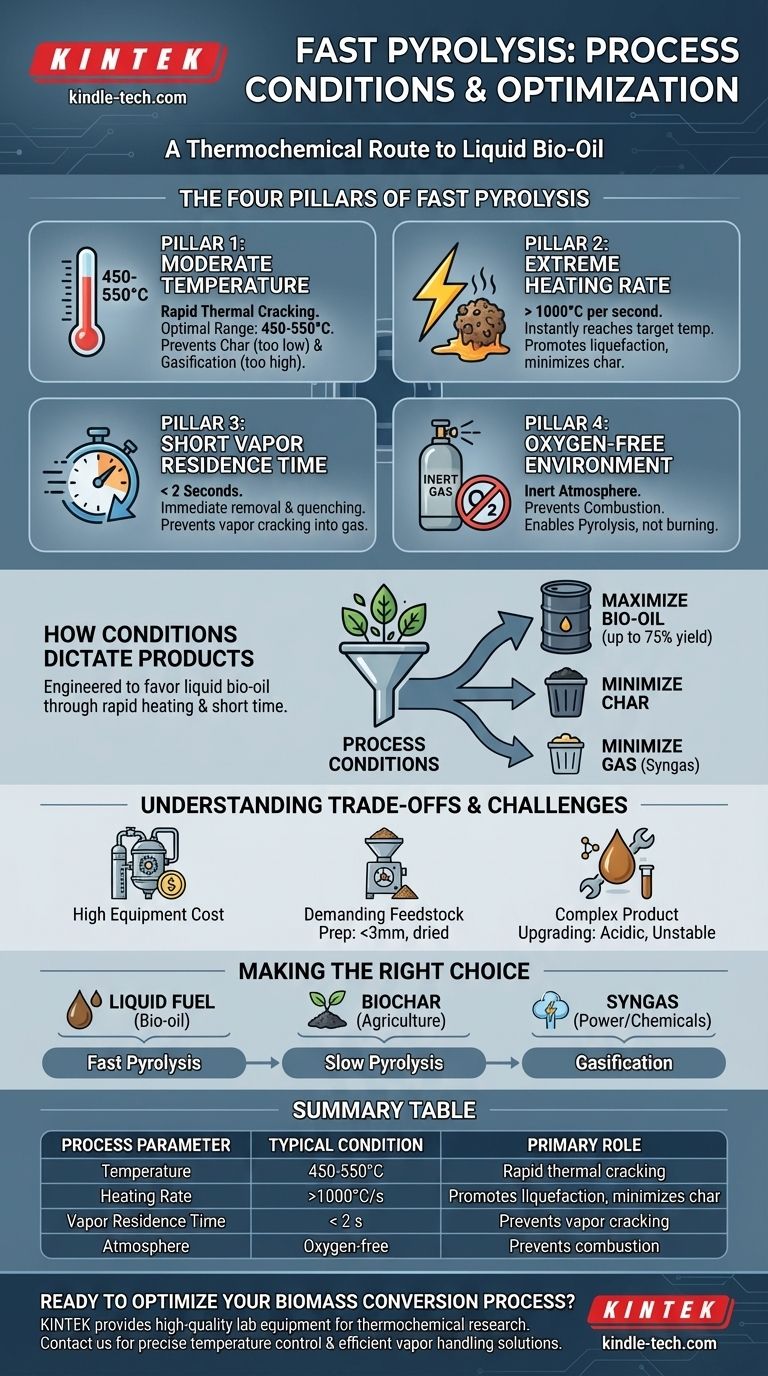
At its core, fast pyrolysis is a thermochemical process defined by three critical conditions designed to rapidly decompose biomass into a liquid fuel. These conditions are a moderate temperature of approximately 500°C, an extremely high heating rate, and a very short vapor residence time of less than two seconds, all conducted in the absence of oxygen.
Fast pyrolysis is not just about heating biomass; it is a precisely engineered process where speed is paramount. The specific conditions are designed to maximize the yield of liquid bio-oil by vaporizing the biomass and immediately quenching the vapors before they can break down into less valuable gases.

The Three Pillars of Fast Pyrolysis
To achieve the primary goal of maximizing liquid bio-oil, the process must be controlled across three key parameters. Each one plays a distinct and critical role in dictating the final product distribution.
Pillar 1: Moderate Temperature (~500°C)
The process is operated in a temperature range of 450-550°C. This temperature is high enough to provide the energy needed for the rapid and complete thermal cracking of the biomass structure—cellulose, hemicellulose, and lignin.
Operating significantly below this range would slow the reaction, favoring the production of solid char (slow pyrolysis). Operating much higher would favor the secondary cracking of vapors into syngas (gasification).
Pillar 2: Extremely High Heating Rate
The biomass particles must be heated at an extremely high rate, often exceeding 1000°C per second. This is perhaps the most defining characteristic of fast pyrolysis.
This rapid energy transfer ensures the entire particle reaches the target temperature almost instantly. It promotes liquefaction pathways and prevents slower reactions that form excess char, effectively "melting" the biomass into vapors.
Pillar 3: Short Vapor Residence Time (< 2 seconds)
Once the biomass breaks down into hot vapors, they must be removed from the hot reactor zone immediately, typically in under two seconds.
These initial vapors are the valuable precursors to bio-oil. If left in the hot zone, they will continue to react and "crack" into smaller, non-condensable molecules like methane and hydrogen. Rapid removal and quenching are therefore essential to capture and condense them into a liquid.
Pillar 4: An Oxygen-Free Environment
All pyrolysis processes, by definition, occur in the absence of an oxidizing agent like oxygen. An inert gas, such as nitrogen, is used to purge the reactor.
This is crucial because the presence of oxygen would lead to combustion (burning) rather than pyrolysis, producing mainly carbon dioxide, water, and ash instead of the desired fuel products.
How Conditions Dictate Products
The process conditions are a deliberate choice to steer the chemical reactions toward a specific outcome. The main product of fast pyrolysis is bio-oil, a liquid fuel, which can account for up to 75% of the product mass.
Maximizing Bio-Oil
The combination of high heating rates and short residence times is engineered specifically to maximize this liquid yield. The process is optimized to create vapors and then immediately cool and condense them, locking in their value as a liquid.
Minimizing Char and Gas
The same conditions that favor bio-oil actively suppress the formation of other products. The fast heating rates minimize char formation, and the short vapor residence times prevent the bio-oil vapors from degrading into syngas. The non-condensable gases that are produced can be captured and burned to provide heat for the reactor, helping to sustain the process.
Understanding the Trade-offs
While effective, the precise conditions of fast pyrolysis create significant engineering and economic challenges.
High Equipment Cost
Achieving extremely high heating rates and rapid vapor quenching requires specialized and complex reactors, such as circulating fluidized bed or ablative reactors. This sophisticated equipment carries a high capital cost.
Demanding Feedstock Preparation
The process works best with small, dry biomass particles (typically <3 mm). This means the raw biomass (like wood chips or agricultural waste) must be dried and finely ground before being fed into the reactor, which adds significant energy and operational costs.
Complex Product Upgrading
The resulting bio-oil is not a "drop-in" replacement for petroleum fuels. It is acidic, unstable, and has a high water and oxygen content. It must undergo further costly upgrading processes, like hydrotreating, before it can be used in conventional engines or refineries.
Making the Right Choice for Your Goal
Understanding the process conditions allows you to select the right technology for your desired outcome.
- If your primary focus is maximizing liquid fuel production: You must adhere strictly to the fast pyrolysis conditions of high heating rates and short residence times.
- If your primary focus is producing biochar for agriculture: You should choose slow pyrolysis, which uses much lower heating rates and very long residence times (hours to days).
- If your primary focus is generating syngas for power or chemical synthesis: You should opt for gasification, which involves higher temperatures and the controlled introduction of oxygen.
Ultimately, mastering the process conditions is the key to unlocking the specific value contained within biomass.
Summary Table:
| Process Parameter | Typical Condition | Primary Role |
|---|---|---|
| Temperature | 450-550°C | Rapid thermal cracking of biomass |
| Heating Rate | >1000°C/second | Promotes liquefaction, minimizes char |
| Vapor Residence Time | < 2 seconds | Prevents vapor cracking into gas |
| Atmosphere | Oxygen-free (inert gas) | Prevents combustion, enables pyrolysis |
Ready to optimize your biomass conversion process?
At KINTEK, we specialize in providing high-quality lab equipment and consumables for advanced thermochemical research, including pyrolysis. Whether you are developing new bio-oil production methods or scaling up your process, our reliable solutions can help you achieve precise temperature control and efficient vapor handling.
Contact our experts today to discuss how KINTEK can support your laboratory's biomass conversion and energy research needs.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- What are the components of a pyrolysis machine? A Complete Breakdown of the Core System
- What is the temperature of calcination decomposition? A Guide to Material-Specific Ranges
- What is the operating temperature of pyrolysis? Master the Key to Biochar, Bio-oil, and Syngas Production
- What are the uses of pyrolysis products? Unlock Value from Bio-Oil, Biochar, and Syngas
- What are the advantages of pyrolysis process? Turn Waste into Valuable Resources and Energy
- Does pyrolysis use a lot of energy? Achieve Net Energy Positive Waste Conversion
- What is the process of rotary calciner? Achieve Uniform Thermal Treatment for Bulk Solids
- What is the operational mechanism of a high-temperature pyrolysis furnace? Expert Guide to Coconut Shell Carbonization



















