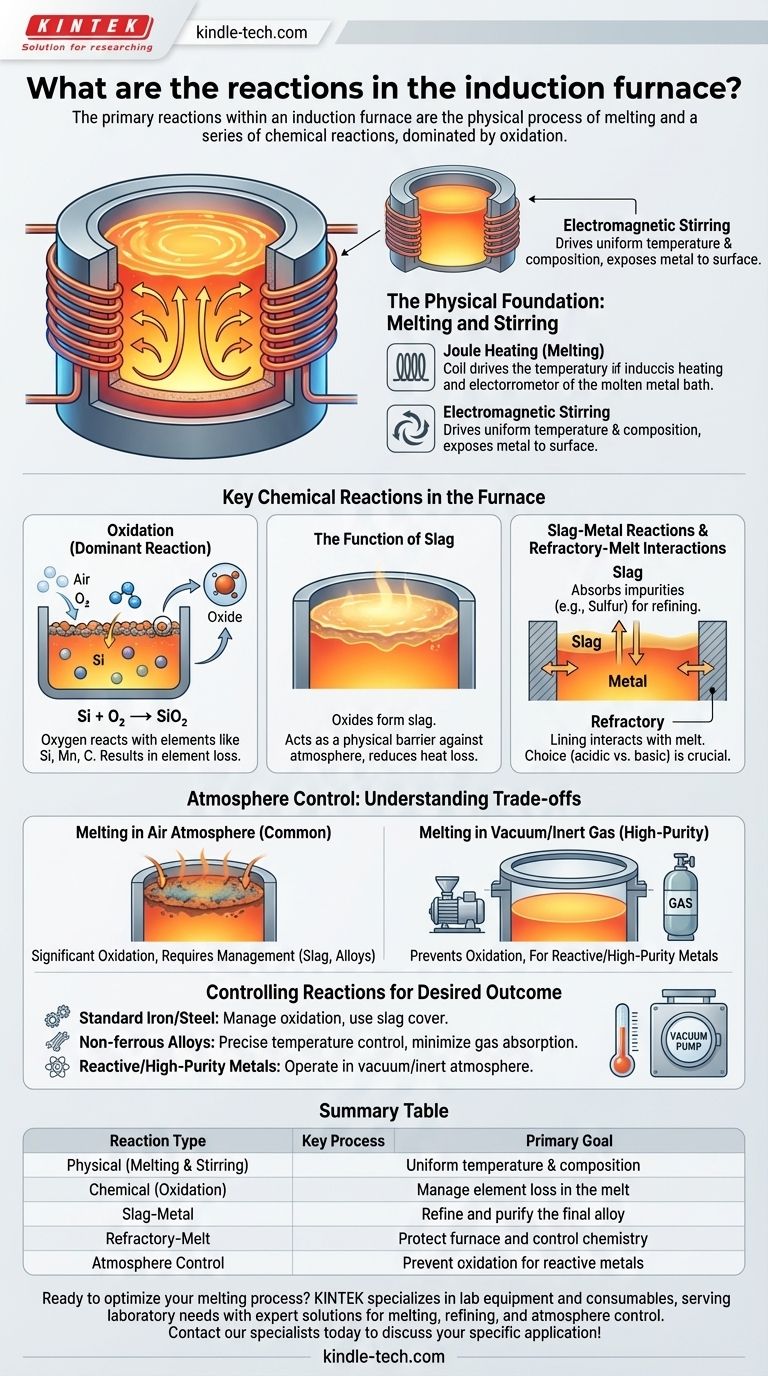
The primary reactions within an induction furnace are the physical process of melting and a series of chemical reactions, dominated by oxidation. While the furnace's main purpose is to change the state of metal from solid to liquid, the intense heat and electromagnetic stirring create a highly reactive environment where the molten metal interacts with the atmosphere, the furnace lining, and any slag present.
An induction furnace is not merely a melting pot; it is an active metallurgical reactor. The key is understanding that the furnace's electromagnetic stirring is the engine that drives crucial chemical reactions, which must be controlled to achieve the desired final metal chemistry.

The Physical Foundation: Melting and Stirring
The chemical reactions in an induction furnace are directly influenced by its unique physical operation.
The Melting Process
An induction furnace uses a powerful alternating current in a copper coil to create a fluctuating magnetic field. This field induces strong electrical currents (eddy currents) within the metal charge, and the metal's electrical resistance generates intense heat (Joule heating), causing it to melt.
The Role of Electromagnetic Stirring
The same magnetic forces that heat the metal also create a vigorous stirring motion. This force pushes the molten metal upwards in the center and down along the sides.
This stirring is not just a side effect; it is a critical process driver. It ensures a uniform temperature and chemical composition throughout the melt, but it also constantly exposes new, unreacted metal to the surface, accelerating interactions with the atmosphere and slag.
Key Chemical Reactions in the Furnace
Once the metal is molten, it becomes a site for several important chemical reactions.
Oxidation: The Dominant Reaction
When melting in an open-air atmosphere, oxygen is the most reactive element present. It readily combines with elements in the molten bath, especially those with a high affinity for oxygen like silicon, manganese, and carbon (in ferrous melts).
For example, silicon in an iron melt will react with oxygen from the air to form silicon dioxide:
Si + O₂ → SiO₂
This oxidation results in a loss of these elements from the melt, which must be accounted for in the initial charge calculation to meet final chemical specifications.
The Function of Slag
The oxides formed during melting, like silicon dioxide, are typically less dense than the molten metal. They float to the surface to form a liquid layer known as slag.
This slag layer is not simply waste. It acts as a physical barrier, protecting the molten metal from further oxidation from the atmosphere and reducing heat loss.
Slag-Metal Reactions
The slag itself is a chemically reactive medium. It can be used to refine the metal by absorbing impurities. For instance, a properly formulated slag can draw sulfur out of a steel melt, a critical step for improving its mechanical properties.
The composition of the slag (its basicity or acidity) is carefully controlled to optimize this refining process and ensure it does not attack the furnace lining.
Refractory-Melt Interactions
The furnace is lined with a heat-resistant material called a refractory. This lining is not perfectly inert and can interact with the melt.
An acidic refractory (silica-based) can be eroded by a basic slag and may even release small amounts of silicon into the melt. Conversely, a basic refractory (magnesia-based) is used for melts that require a basic slag for refining. The choice of refractory is a fundamental decision that dictates the type of chemistry you can perform.
Understanding the Trade-offs: Atmosphere vs. Vacuum
The reference to operating in an atmosphere or vacuum highlights a critical choice that directly controls the furnace's reactive environment.
Melting in an Air Atmosphere
This is the most common and cost-effective method. However, the trade-off is significant oxidation. Operators must manage this by controlling the temperature, using a protective slag cover, and adjusting the initial alloy additions to compensate for expected losses.
Melting in a Vacuum or Inert Gas
For highly reactive metals like titanium or high-purity superalloys, melting must occur in a vacuum or an inert atmosphere (like argon). This is far more complex and expensive but is the only way to prevent the detrimental oxidation reactions that would compromise the metal's integrity.
Controlling Reactions for Your Desired Outcome
Understanding these reactions allows you to control the final product. Your approach will depend entirely on your goal.
- If your primary focus is producing standard iron or steel castings: Your main goal is managing oxidation by using a proper slag cover and adjusting alloy additions to compensate for predictable element loss.
- If your primary focus is melting high-value non-ferrous alloys (e.g., copper, aluminum): Precise temperature control is paramount to minimize both oxidation and the absorption of detrimental gases like hydrogen, which causes porosity.
- If your primary focus is producing reactive or high-purity metals (e.g., superalloys): You must operate in a vacuum or inert atmosphere to prevent any unwanted chemical reactions with the air.
By mastering these interactions, the induction furnace is transformed from a simple melter into a precise metallurgical tool.
Summary Table:
| Reaction Type | Key Process | Primary Goal |
|---|---|---|
| Physical | Melting & Electromagnetic Stirring | Uniform temperature and composition |
| Chemical (Oxidation) | Metal reacts with oxygen (e.g., Si + O₂ → SiO₂) | Manage element loss in the melt |
| Slag-Metal | Slag absorbs impurities (e.g., sulfur) from the metal | Refine and purify the final alloy |
| Refractory-Melt | Interaction between the furnace lining and the melt/slag | Protect furnace and control chemistry |
| Atmosphere Control | Melting in air vs. vacuum/inert gas | Prevent oxidation for reactive metals |
Ready to optimize your melting process? The reactions in your induction furnace are the key to your final product's quality and consistency. KINTEK specializes in lab equipment and consumables, serving laboratory needs with expert solutions for melting, refining, and atmosphere control. Let our expertise help you achieve precise metallurgical outcomes. Contact our specialists today to discuss your specific application!
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- 1800℃ Muffle Oven Furnace for Laboratory
- Lab-Scale Vacuum Induction Melting Furnace
People Also Ask
- What is a tubular furnace used for? Precision Heating for Material Synthesis & Analysis
- How does a high-temperature tube furnace facilitate the phase transformation of alumina products? Master Thermal Control
- What is the technical value of using a quartz tube reaction chamber for static corrosion testing? Achieve Precision.
- How do a quartz tube reactor and atmosphere furnace collaborate in Co@NC pyrolysis? Master Precision Synthesis
- What precautions should be taken when using a tube furnace? Ensure Safe, Effective High-Temperature Processing



















