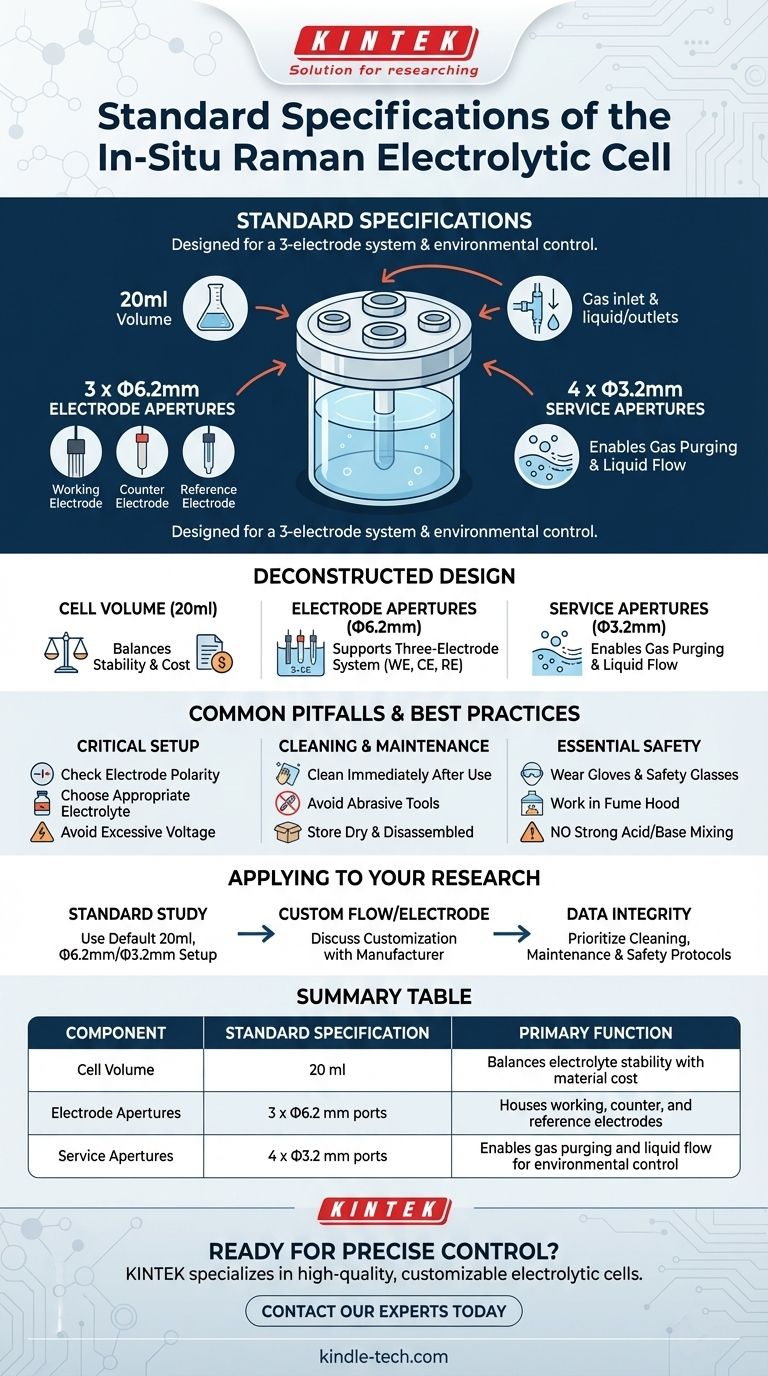
The standard specifications for an in-situ Raman electrolytic cell are a volume of 20ml and a specific set of apertures designed for a three-electrode system. These typically include three larger openings of Φ6.2mm for the electrodes and four smaller openings of Φ3.2mm for gas and liquid exchange.
While the standard dimensions provide a baseline, the true purpose of this design is to create a controlled environment for simultaneous electrochemical manipulation and spectroscopic observation. Understanding the function of each component is more critical than the exact specifications, as customization is common.

Deconstructing the Cell's Design
The specifications of an in-situ Raman cell are not arbitrary. They are purposefully designed to support the complex requirements of spectroelectrochemistry, balancing the needs of the electrochemical setup with the optical demands of the Raman spectrometer.
The Standard Volume (20ml)
The 20ml volume is a common standard because it offers a practical balance. It is large enough to prevent rapid depletion of reactants or saturation with products during an experiment, ensuring the bulk electrolyte remains relatively stable.
At the same time, it is small enough to be cost-effective, minimizing the amount of expensive electrolyte, solvents, or novel chemical species required for your investigation.
The Electrode Apertures (Φ6.2mm)
The cell is designed for a three-electrode system, and the three Φ6.2mm ports accommodate this setup.
One port is for the Working Electrode, the surface where the reaction of interest occurs and which is positioned in the Raman laser's focal path.
A second port holds the Counter Electrode (or auxiliary electrode), which completes the circuit and balances the current from the working electrode.
The third port is for the Reference Electrode, which provides a stable potential to measure the working electrode against. This is often accomplished using a Luggin capillary to minimize iR drop and ensure accurate potential control.
The Service Apertures (Φ3.2mm)
The four smaller Φ3.2mm ports provide essential utility for controlling the experimental environment.
These are used for the inlet and outlet of gas or liquid. Common uses include purging the electrolyte with an inert gas (like nitrogen or argon) to remove dissolved oxygen or establishing a flow-cell configuration for continuous analysis.
Common Pitfalls and Best Practices
Successful in-situ analysis depends on meticulous setup and handling. Mistakes can easily compromise your data, damage the equipment, or create safety hazards.
Critical Setup Procedures
Before starting, ensure the correct electrode polarity. Reversing the anode and cathode connections can lead to unintended reactions and invalidate your results.
Choose an electrolyte appropriate for your experiment. An unsuitable electrolyte can cause unwanted side reactions that obscure the process you intend to study.
Avoid applying excessively high voltage. This can cause the electrolyte to decompose or lead to irreversible damage to the electrode surfaces.
Proper Cleaning and Maintenance
Clean the cell and electrodes immediately after every experiment to prevent residue from hardening and contaminating future work. A standard cleaning protocol involves wiping with acetone, rinsing with ethanol, and finishing with a rinse of high-purity (18.2 MΩ·cm) ultrapure water.
Never use metal brushes or abrasive tools that could scratch the optical window or electrode surfaces.
For storage, ensure all components are completely dry and keep them in a moisture-free environment. For long-term storage, disassemble the cell.
Essential Safety Protocols
Always handle these cells with care due to their complex and often delicate construction.
Wear protective gloves and safety glasses, especially when working with corrosive electrolytes. Conduct your experiments in a well-ventilated fume hood to avoid inhaling any harmful gases produced.
Never mix strong acids and bases (e.g., HNO₃ + NaOH) inside the cell for cleaning, as this can generate a dangerous exothermic reaction.
How to Apply This to Your Research
Your experimental goals should dictate how you approach the cell's specifications and use.
- If your primary focus is conducting a standard electrochemical study: The 20ml cell with its default Φ6.2mm and Φ3.2mm apertures is likely the ideal starting point.
- If your primary focus is developing a custom flow system or using non-standard electrodes: You should plan to discuss customizing the number, size, and position of the apertures with the manufacturer.
- If your primary focus is ensuring data integrity and long-term use: Prioritize mastering the rigorous cleaning, maintenance, and safety protocols above all else.
Ultimately, mastering the use of this tool is about controlling the environment to isolate the reaction you wish to observe.
Summary Table:
| Component | Standard Specification | Primary Function |
|---|---|---|
| Cell Volume | 20 ml | Balances electrolyte stability with material cost |
| Electrode Apertures | 3 x Φ6.2 mm ports | Houses working, counter, and reference electrodes |
| Service Apertures | 4 x Φ3.2 mm ports | Enables gas purging and liquid flow for environmental control |
Ready to achieve precise control in your spectroelectrochemistry experiments?
The standard in-situ Raman cell is a starting point, but your research is unique. KINTEK specializes in high-quality lab equipment and consumables, including customizable electrolytic cells designed for reliability and optimal optical performance.
Whether you need a standard configuration or a custom solution tailored to your specific electrode setup and flow requirements, our expertise ensures your lab is equipped for success.
Contact our experts today to discuss your application and find the perfect cell for your research needs.
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
- H Type Electrolytic Cell Triple Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- What materials are used for the caps of the sealed and non-sealed electrolysis cells? PTFE vs. POM Explained
- How are ion-exchange membranes selected for H-type electrolytic cells? Ensure Optimal Ion Transport and Reaction Purity
- What is the proper way to handle the glass components of the electrolytic cell? Ensure Safe and Accurate Experiments
- What are the primary functions of a high-performance electrolytic cell in the eCO2R process? Optimize Your Lab Results
- What is the function of a three-electrode electrolytic cell? Enhance EIS Accuracy for Polyester Coating Evaluation
- What is the difference between a voltaic cell and an electrochemical cell? Understand the Two Types of Energy Conversion
- What is the operating principle of a flat plate corrosion electrolytic cell? A Guide to Controlled Materials Testing
- What are the components and their respective functions in a flat plate corrosion electrolytic cell system? A Guide to Precise Corrosion Measurement



















