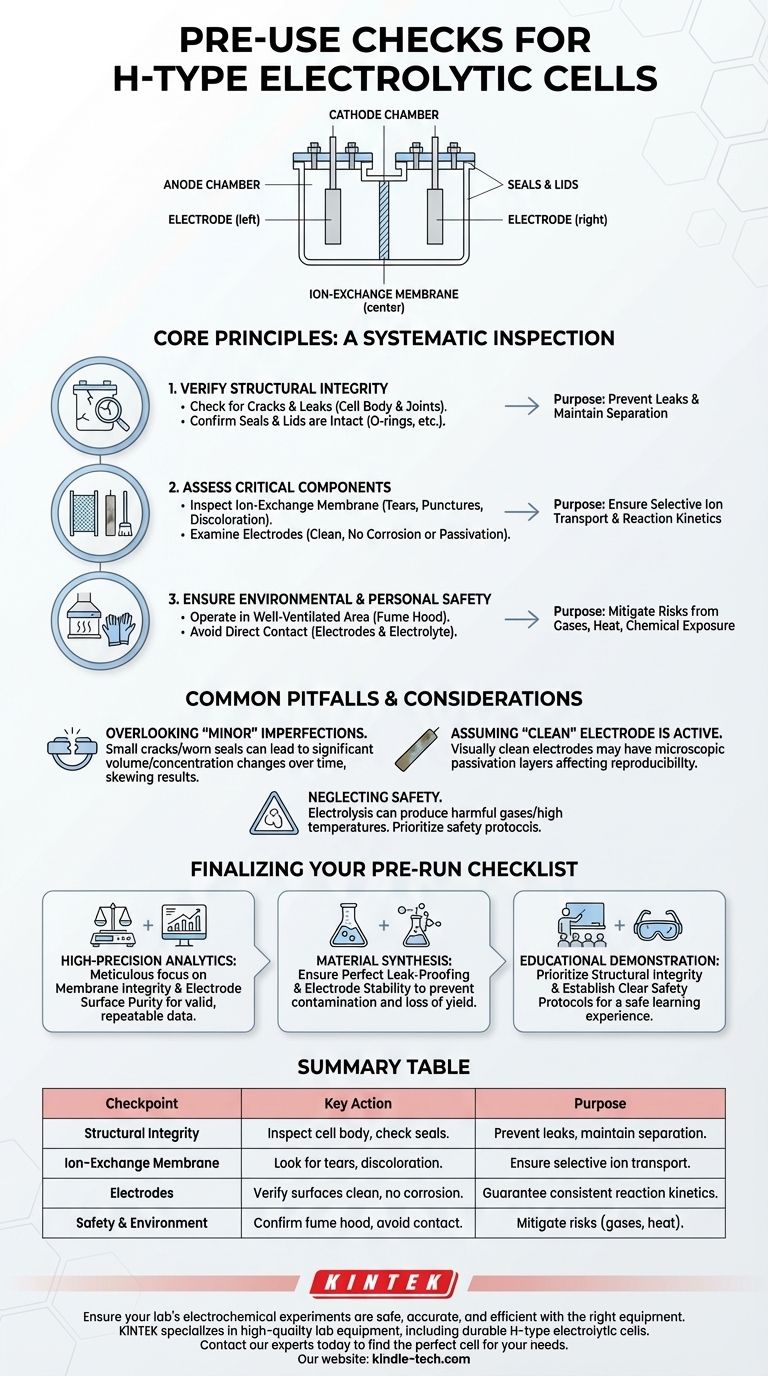
Before any experiment, a systematic inspection of your H-type electrolytic cell is non-negotiable. This process involves more than a quick glance; it requires a detailed check of all components, including the cell body, anode and cathode chambers, electrodes, and especially the ion-exchange membrane. You must verify that every part is clean, undamaged, and correctly assembled to ensure the safety and validity of your results.
The purpose of a pre-use check is to guarantee the complete physical and chemical separation between the cell's two chambers. A failure at any point—from a hairline crack to a degraded membrane—not only compromises the integrity of your experiment but also introduces significant safety risks.

The Core Principles of a Pre-Use Inspection
A thorough inspection can be broken down into three key areas: the cell's overall structure, the condition of its active components, and the final assembly.
Verifying Structural Integrity
The glass or polymer body of the cell is your first line of defense against leaks and experimental failure.
Check for Cracks and Leaks
Even a hairline crack can compromise the experiment by allowing electrolyte to leak out or air to leak in. Carefully inspect the entire cell body, especially around joints and ports, for any signs of damage.
Confirm Seals and Lids are Intact
Ensure all sealing rings (like O-rings) are present, pliable, and free of nicks or signs of aging. A faulty seal is a primary cause of leaks that can alter electrolyte concentrations and ruin data.
Assessing Critical Component Condition
The membrane and electrodes are the heart of the electrochemical process. Their condition directly dictates the performance and outcome of your experiment.
Inspect the Ion-Exchange Membrane
The membrane's sole purpose is to allow specific ions to pass while preventing the bulk mixing of the anolyte and catholyte. Check it carefully for any tears, punctures, or discoloration that could indicate chemical degradation or aging. A compromised membrane invalidates the entire premise of an H-type cell.
Examine the Electrodes
The surface of your anode and cathode is where the reaction happens. Ensure the surfaces are clean and free from corrosion, pitting, or any passive layer that might have formed from previous use. Contaminated or damaged electrodes lead to inconsistent results and altered reaction kinetics. In many cases, cleaning or polishing may be required before use.
Common Pitfalls and Safety Considerations
Failing to perform these checks can lead to wasted time, inaccurate data, and serious safety hazards.
Overlooking "Minor" Imperfections
A small crack or a slightly worn seal may seem insignificant, but over the course of an hours-long experiment, it can lead to a significant change in volume or concentration, skewing your results. Treat every imperfection as a potential point of failure.
Assuming a "Clean" Electrode is Active
An electrode can appear visually clean but still have a microscopic, electrically insulating passivation layer on its surface. If your results are not reproducible, unexpected electrode surface conditions are a common culprit.
Neglecting Environmental and Personal Safety
Remember that electrolysis can produce high temperatures or harmful gases. Always operate the cell in a well-ventilated area, such as a fume hood. To prevent electric shock, chemical burns, or poisoning, you must avoid any direct physical contact with the electrodes and electrolyte during operation.
Finalizing Your Pre-Run Checklist
Your level of scrutiny should match the demands of your experiment.
- If your primary focus is high-precision analytics: You must be meticulous about membrane integrity and electrode surface purity to ensure valid, repeatable data.
- If your primary focus is material synthesis: Ensure the cell is perfectly leak-proof and electrodes are stable to prevent contamination of your product and loss of yield.
- If your primary focus is educational demonstration: Prioritize confirming the overall structural integrity and establishing clear safety protocols to ensure a safe and effective learning experience.
A disciplined pre-use check transforms your electrolytic cell from a piece of glassware into a reliable scientific instrument.
Summary Table:
| Checkpoint | Key Action | Purpose |
|---|---|---|
| Structural Integrity | Inspect cell body for cracks; check seals and lids. | Prevent leaks and maintain electrolyte separation. |
| Ion-Exchange Membrane | Look for tears, punctures, or discoloration. | Ensure selective ion transport and prevent chamber mixing. |
| Electrodes | Verify surfaces are clean, free of corrosion or passivation. | Guarantee consistent reaction kinetics and reproducible data. |
| Safety & Environment | Confirm operation in a fume hood; avoid direct contact. | Mitigate risks from gases, heat, or chemical exposure. |
Ensure your lab's electrochemical experiments are safe, accurate, and efficient with the right equipment. KINTEK specializes in high-quality lab equipment and consumables, including durable H-type electrolytic cells designed for reliable performance. Our products help you achieve precise separations and reproducible results, minimizing the risk of experimental failure.
Contact our experts today to find the perfect electrolytic cell for your research or synthesis needs!
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
People Also Ask
- What are the key safety operation guidelines for using the H-type electrolytic cell? Best Practices for Your Lab
- What is the function of an H-type exchangeable membrane electrolytic cell? Master Precise Reaction Control
- How should failures or malfunctions of an H-type electrolytic cell be handled? A Guide to Safe and Effective Troubleshooting
- What should be observed during an experiment with the H-type electrolytic cell? Key Monitoring for Precise Results
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry



















