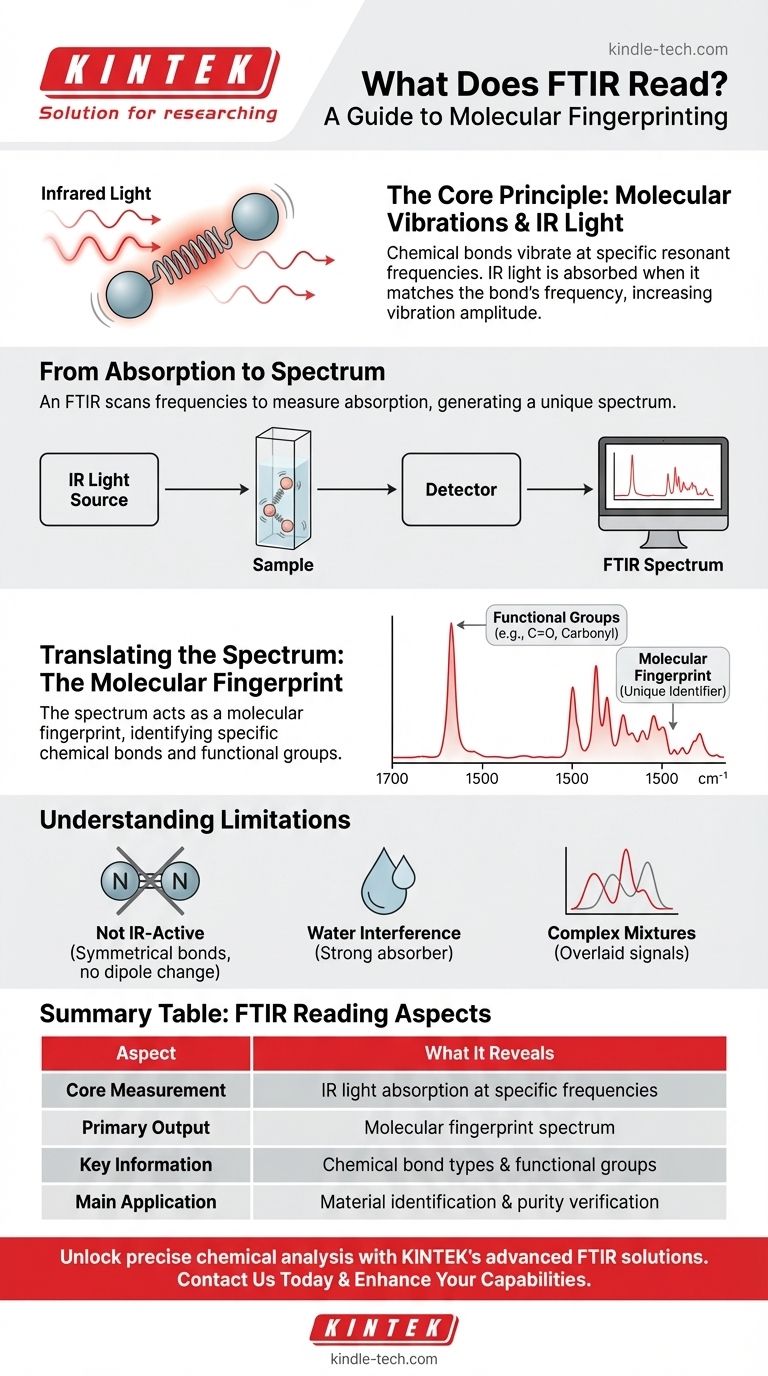
At its core, an FTIR spectrometer reads how much infrared light a sample absorbs at various frequencies. This process generates a unique spectrum, which acts as a "molecular fingerprint." By analyzing this fingerprint, scientists can identify the chemical bonds within a material, allowing them to characterize new substances or verify the identity and purity of known samples.
The crucial insight is that FTIR does not identify chemicals directly. Instead, it measures the vibrational energy of chemical bonds. By interpreting which specific frequencies of light are absorbed, we can deduce the types of bonds present and ultimately understand the molecule's structure.

The Principle Behind the Reading: Molecular Vibrations
To understand what an FTIR "reads," you must first understand how molecules behave. The instrument is designed to measure a fundamental property of molecular structure.
Chemical Bonds Aren't Static
Chemical bonds connecting atoms within a molecule are not rigid rods. They behave more like tiny springs that are constantly in motion, capable of stretching, bending, and vibrating in various ways.
A Resonant Frequency
Each type of chemical bond (like a Carbon-Hydrogen bond or a Carbon-Oxygen double bond) has a specific, natural frequency at which it prefers to vibrate. This is determined by the mass of the atoms and the strength of the bond connecting them.
Infrared Light as an Energy Source
When infrared light is passed through a sample, the molecule will absorb the light's energy only if the frequency of the light matches the natural vibrational frequency of a bond. This energy absorption causes the bond's vibration to increase in amplitude.
From Absorption to a Spectrum
An FTIR instrument scans a wide range of infrared frequencies through the sample and detects how much light passes through at each frequency. The resulting plot, known as an FTIR spectrum, shows the frequencies where light was absorbed. This spectrum is the direct "reading" from the instrument.
Translating the FTIR Spectrum into Chemical Information
The raw spectrum of absorption peaks is just data. The real power lies in translating this data into meaningful chemical knowledge.
The "Molecular Fingerprint"
The full spectrum of absorption peaks is unique to a specific molecule. The combination and intensity of these peaks serve as an unambiguous identifier, much like a human fingerprint. By comparing a sample's spectrum to a library of known spectra, one can quickly identify an unknown compound.
Identifying Functional Groups
Even without a full library match, the spectrum is incredibly useful. Specific regions of the infrared spectrum correspond to the vibrations of specific functional groups—the building blocks of organic molecules. For example, a strong absorption peak around 1700 cm⁻¹ almost always indicates the presence of a carbonyl (C=O) group.
Understanding the Limitations
While powerful, the FTIR technique has limitations that are important to recognize for accurate interpretation.
Not All Bonds are IR-Active
For a bond to absorb infrared light, its vibration must cause a change in the molecule's dipole moment. Symmetrical bonds, such as the N≡N triple bond in nitrogen gas or the O=O double bond in oxygen gas, do not have a changing dipole moment as they vibrate. Consequently, they do not absorb IR light and are invisible to FTIR.
The Impact of Water
Water is a very strong absorber of infrared light and has broad absorption peaks. The presence of water in a sample can easily obscure the peaks from the substance of interest, making analysis difficult or impossible if not properly removed or accounted for.
Complexity of Mixtures
Analyzing a simple, pure compound is straightforward. However, for complex mixtures, the spectra of all the components are overlaid on top of one another. Separating and identifying individual substances from this combined spectrum can be challenging and often requires more advanced analytical techniques.
Making the Right Choice for Your Goal
The way you interpret an FTIR reading depends entirely on your analytical objective.
- If your primary focus is identifying an unknown pure substance: Compare the entire fingerprint region (typically below 1500 cm⁻¹) of your sample's spectrum against a spectral library for a direct match.
- If your primary focus is verifying a chemical transformation: Look for the disappearance of peaks corresponding to reactant functional groups and the appearance of new peaks for product functional groups.
- If your primary focus is assessing material purity or degradation: Compare your sample's spectrum to that of a pure reference standard, looking for additional or unexpected peaks that indicate impurities or chemical breakdown.
By understanding that an FTIR reading is a direct map of a molecule's vibrational energies, you can translate a simple spectrum into powerful and actionable chemical insights.
Summary Table:
| FTIR Reading Aspect | What It Reveals |
|---|---|
| Core Measurement | Infrared light absorption at specific frequencies |
| Primary Output | Molecular fingerprint spectrum |
| Key Information | Chemical bond types and functional groups |
| Main Application | Material identification and purity verification |
| Limitations | Not IR-active bonds, water interference, complex mixtures |
Ready to unlock precise chemical analysis for your laboratory?
At KINTEK, we specialize in providing advanced FTIR spectrometers and lab equipment that deliver accurate molecular fingerprints for your research and quality control needs. Whether you're identifying unknown substances, verifying chemical transformations, or assessing material purity, our solutions are designed to give you clear, actionable insights.
Let our experts help you enhance your analytical capabilities:
Contact us today to discuss your specific laboratory requirements and discover how KINTEK's precision instruments can advance your work.
Visual Guide
Related Products
- Square Lab Press Mold for Laboratory Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Laboratory High Pressure Horizontal Autoclave Steam Sterilizer for Lab Use
- Optical Window Glass Substrate Wafer Barium Fluoride BaF2 Substrate Window
- Thin-Layer Spectral Electrolysis Electrochemical Cell
People Also Ask
- What is the flow rate of a filter press? Mastering the Dynamic Filtration Cycle
- What are the heat treatment processes in the heat treatment of steel? Master the Methods for Superior Material Properties
- Does heat treatment increase strength? Unlock Maximum Metal Performance for Your Components
- What are the precautions to be taken during blending of metal powders? Ensure Safety and Quality in Your Lab
- What is the difference between melting and sintering? A Guide to Solid-State vs. Liquid-State Processes
- What essential properties are required in a good refractory? Achieve Optimal Performance & Efficiency
- Why is a laboratory-grade forced air drying oven required for alloy chip moisture analysis? Ensure Data Precision
- Does nanomaterials have potential hazards to human health? Understanding the Risks and Safe Handling



















