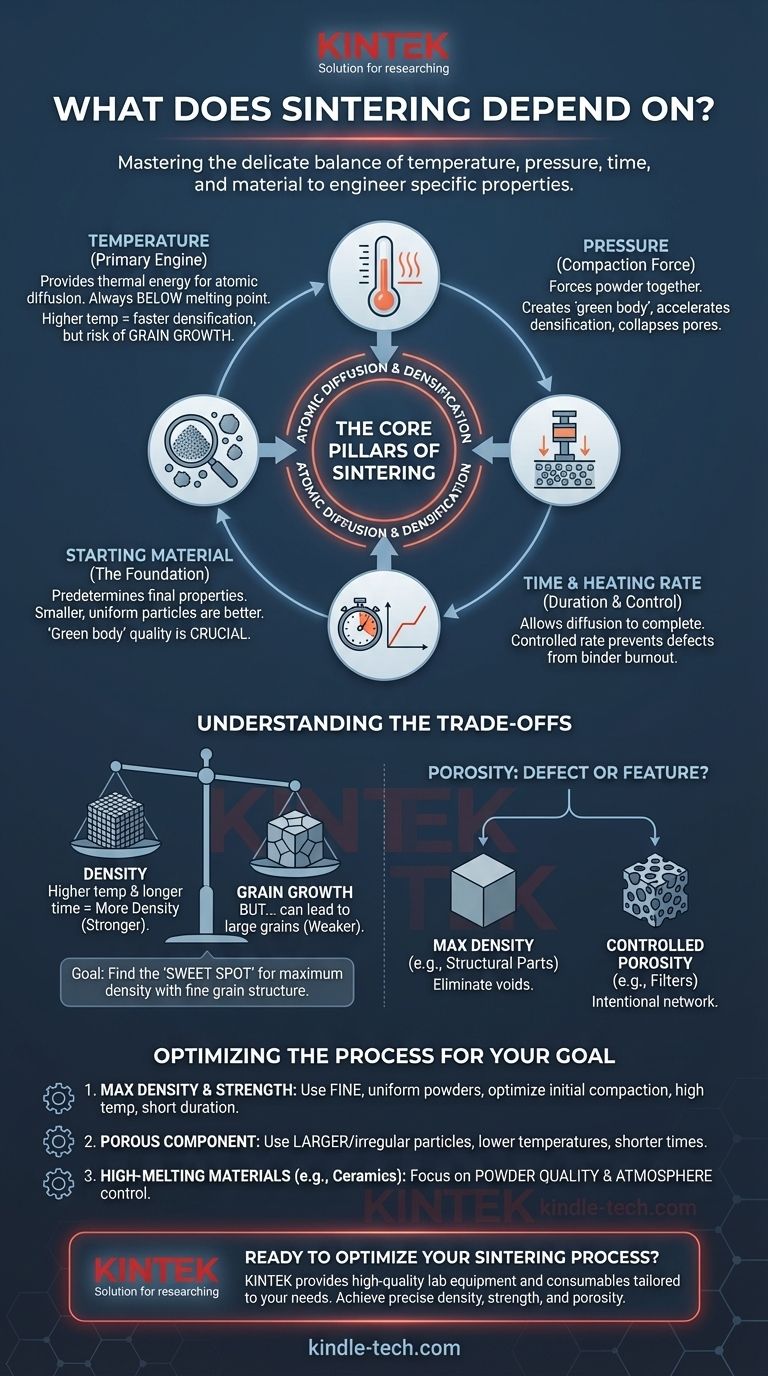
At its core, the success of sintering depends on four key variables: temperature, pressure, time, and the characteristics of the starting material. These factors are not independent; they work together to control the atomic diffusion process that fuses loose powder into a solid, dense object without melting it. Understanding how to manipulate these variables is the key to engineering a final product with specific properties like strength, density, and porosity.
Sintering is a delicate balancing act. The goal is to apply just enough thermal energy and pressure over a specific duration to bond particles together, eliminating voids. The entire process is fundamentally governed by the initial state of the material and the desired properties of the final component.

The Core Pillars of Sintering
Sintering is driven by a few primary physical parameters. Adjusting these levers allows you to control the rate and extent of densification.
The Role of Temperature
Temperature is the primary engine of sintering. It provides the thermal energy necessary for atoms to move and diffuse across the boundaries of adjacent particles.
The temperature is always kept below the material's melting point. This is precisely why sintering is so valuable for materials with extremely high melting points, like tungsten, molybdenum, and many ceramics, which are difficult or impossible to process via melting and casting.
A higher temperature increases the rate of diffusion, leading to faster densification. However, if the temperature is too high, it can cause undesirable grain growth, where smaller grains merge into larger ones, potentially weakening the final part.
The Impact of Pressure
Pressure serves to physically force powder particles closer together. This initial compaction is critical for creating a "green body" with minimal large voids.
During the sintering process itself, external pressure can be applied to accelerate densification. It enhances particle rearrangement and helps collapse pores that might otherwise remain, significantly improving the final density and mechanical properties of the part.
The Element of Time and Heating Rate
Sintering is not an instantaneous process. It requires holding the material at the target temperature for a specific duration to allow diffusion to complete its work.
The heating rate—how quickly the material is brought to the sintering temperature—is also crucial. A slower, more controlled rate allows residual binders or lubricants from the compaction stage to burn off cleanly, preventing defects in the final structure.
Why the Starting Material Is Crucial
The final properties of a sintered part are largely predetermined by the powder you start with. The most sophisticated process cannot fully compensate for poor starting material.
Particle Size and Shape
Smaller, more uniform particles are generally better. They possess a higher surface-area-to-volume ratio, which creates a stronger thermodynamic driving force for diffusion. This results in faster, more complete densification at lower temperatures.
Material Composition and Atmosphere
The intrinsic properties of the material, such as its diffusion coefficient, dictate how readily it will sinter. A homogeneous mixture of powders ensures that densification occurs evenly throughout the part.
Additives like binders are used to hold the green body together before sintering. During heating, these must be burned off. The sintering atmosphere (e.g., the presence of water vapor or inert gas) can be controlled to facilitate this removal and prevent unwanted chemical reactions like oxidation.
Understanding the Trade-offs
Optimizing sintering requires navigating a series of critical trade-offs. The "perfect" set of parameters rarely exists; instead, they are chosen to achieve a specific goal.
Density vs. Grain Growth
This is the central trade-off in sintering. While high temperatures and long sintering times promote higher density by eliminating pores, they also encourage grain growth. Overly large grains can reduce the material's strength and toughness. The goal is often to find the "sweet spot" that achieves maximum density with the finest possible grain structure.
Porosity: Defect or Feature?
While sintering is often used to create a fully dense part, sometimes porosity is a desired feature. Materials for filters or self-lubricating bearings are designed to have a network of interconnected pores.
In these cases, the process is intentionally modified. Using larger particles, lower pressures, or lower temperatures can produce a strong but porous final component.
The Importance of the "Green Body"
The initial compaction step is arguably as important as the sintering itself. If the initial "green body" has low or uneven density with large voids, these defects are extremely difficult to eliminate later. No amount of time or temperature can easily fix a poorly compacted part.
Optimizing the Sintering Process for Your Goal
Your choice of parameters should be directly informed by the intended application of the final component. There is no single "correct" way to sinter; there is only the right way for your objective.
- If your primary focus is maximum density and strength: Use fine, uniform powders, optimize initial compaction, and apply a temperature high enough for rapid diffusion but for a duration short enough to limit excessive grain growth.
- If your primary focus is producing a porous component (e.g., a filter): Use larger or more irregularly shaped particles and lower sintering temperatures or shorter times to intentionally preserve a network of interconnected pores.
- If you are working with high-melting-point materials (e.g., ceramics): Focus heavily on powder quality (fine and pure) and atmospheric control, as you are limited by practical temperature ceilings and must rely on diffusion efficiency.
Mastering sintering is about precisely balancing these interdependent factors to engineer the desired final material properties.
Summary Table:
| Key Variable | Role in Sintering Process | Impact on Final Product |
|---|---|---|
| Temperature | Provides thermal energy for atomic diffusion | Higher temp = faster densification, but risk of grain growth |
| Pressure | Forces particles together, collapses pores | Increases density and mechanical strength |
| Time | Allows diffusion to complete; heating rate affects defect prevention | Longer time = more complete bonding, but potential for grain growth |
| Starting Material | Determines initial particle size, shape, and composition | Fine, uniform particles enable better densification and lower sintering temperatures |
Ready to optimize your sintering process for superior material properties? KINTEK specializes in providing high-quality lab equipment and consumables tailored to your laboratory's sintering needs. Whether you're working with ceramics, metals, or advanced composites, our expertise ensures you achieve the precise density, strength, and porosity required for your application. Contact us today to discuss how our solutions can enhance your sintering outcomes!
Visual Guide
Related Products
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the advantages of vacuum sintering? Achieve Superior Purity, Strength, and Performance
- What is the impact factor of powder metallurgy progress? A 2022 Analysis & Context
- How does a vacuum environment system contribute to the hot pressing sintering of B4C-CeB6? Unlock Peak Ceramic Density
- What conditions does a vacuum hot press provide for Al2O3/ZrO2 sintering? Achieve 1550°C and 30 MPa Densification
- What are the advantages of using a vacuum hot pressing furnace? Achieve 98.9% Density in Al2O3-TiC Laminated Ceramics



















