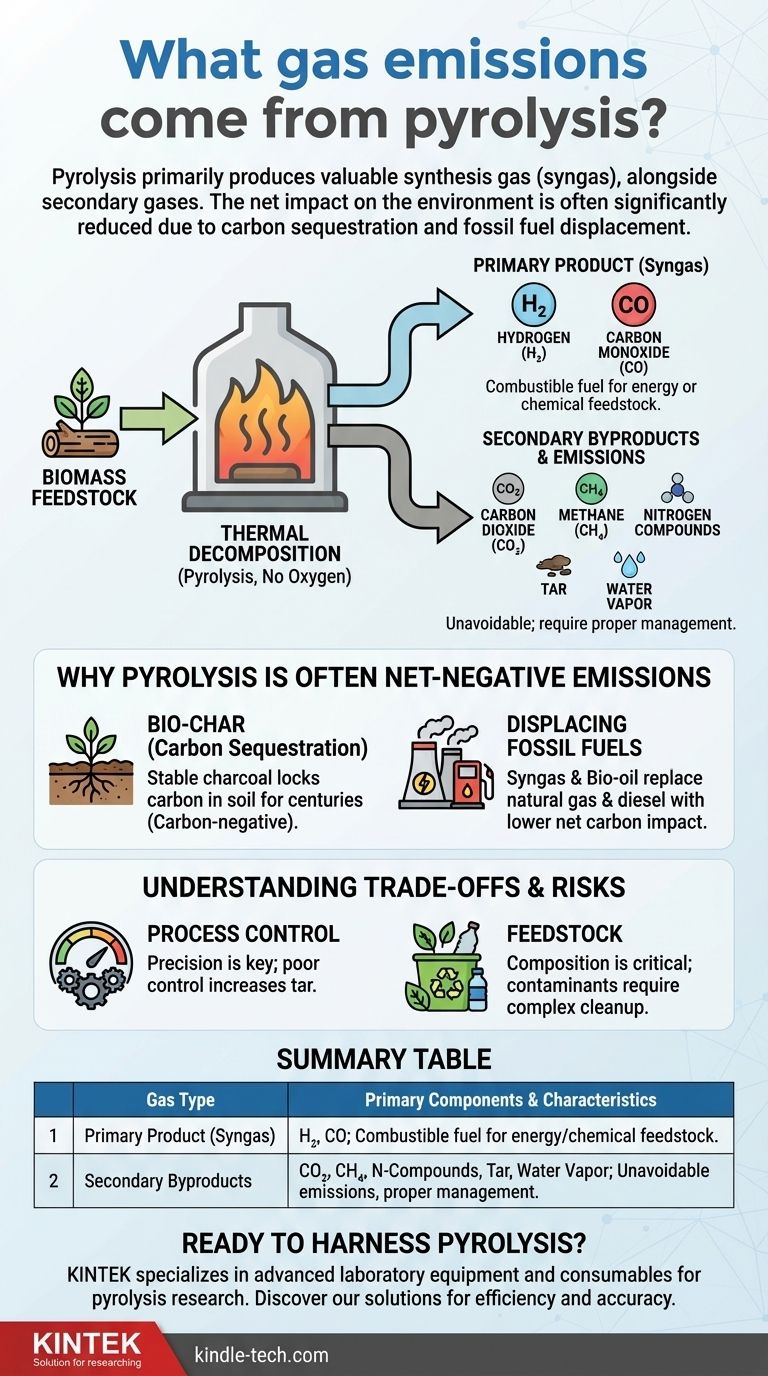
In short, pyrolysis primarily produces a valuable fuel called synthesis gas (syngas), but it also generates secondary gases like carbon dioxide, methane, and nitrogen compounds. The exact composition of these emissions is not fixed; it changes based on the material being processed and the specific conditions of the pyrolysis system.
The critical distinction to understand is between the gases produced during the process and the technology's net impact on the environment. While pyrolysis does emit gases, its ability to convert waste into stable carbon (bio-char) and displace fossil fuels often results in a significant net reduction in overall greenhouse gas emissions.

The Two Classes of Gaseous Products
Pyrolysis is a thermal decomposition process that occurs in the absence of oxygen. The gases it produces can be sorted into two categories: the intended, valuable products and the secondary, unavoidable byproducts.
Primary Product: Synthesis Gas (Syngas)
The main gaseous output is syngas, a combustible mixture that is a valuable energy product. It is primarily composed of hydrogen (H₂) and carbon monoxide (CO).
This gas can be used directly in engines or turbines to generate electricity or heat. It can also serve as a chemical feedstock to produce liquid fuels or other valuable chemicals, reducing our reliance on fossil fuel derivatives.
Secondary Byproducts and Emissions
Alongside syngas, the process generates smaller quantities of other gases and vapor-phase compounds.
These include carbon dioxide (CO₂), methane (CH₄), and various nitrogen compounds if nitrogen is present in the feedstock. Additionally, small amounts of water vapor and tar are also produced. These byproducts must be properly managed.
Why Pyrolysis is Often a Net-Negative Emissions Technology
Understanding the gases released is only half the story. The true environmental impact of pyrolysis comes from how its products interact with the broader carbon cycle.
The Role of Bio-char in Carbon Sequestration
The solid product of biomass pyrolysis is bio-char, a highly stable, charcoal-like substance. This is not just a byproduct; it is a form of captured carbon.
When this bio-char is added to soil, the carbon it contains is locked away, or sequestered, for hundreds or even thousands of years. This effectively removes carbon dioxide from the atmosphere, making the process carbon-negative.
Displacing Fossil Fuels
The energy products of pyrolysis—syngas and a liquid fuel called bio-oil—can directly replace fossil fuels like natural gas and diesel.
The carbon in the biomass feedstock is part of the natural, or biogenic, carbon cycle. Burning the resulting biofuels has a much lower net carbon impact than releasing new, "fossil" carbon into the atmosphere from sources that have been locked underground for millions of years.
Understanding the Trade-offs and Risks
Pyrolysis is a powerful technology, but it is not without complexity. Its environmental benefits depend entirely on proper design and operation.
The Importance of Process Control
The efficiency and cleanliness of pyrolysis hinge on precise control over temperature and processing time. A poorly controlled system can produce excessive amounts of tar, a complex and difficult-to-handle byproduct, reducing the overall energy yield and creating a disposal challenge.
Feedstock Determines Emissions
The composition of the input material, or feedstock, is critical. Processing clean biomass (like wood or crop waste) is straightforward.
However, if the feedstock is contaminated with substances like plastics, heavy metals, or sulfur, these contaminants can be released in the gaseous emissions or concentrated in the char, requiring more complex and costly gas cleanup systems.
How to Evaluate Pyrolysis Emissions
The significance of the emissions depends entirely on your objective. Viewing the process through different lenses helps clarify its true value.
- If your primary focus is waste management: Pyrolysis is an excellent alternative to landfilling, as it avoids the uncontrolled release of methane from decomposition while recovering value from the waste stream.
- If your primary focus is energy production: Pyrolysis generates biofuels that provide a lower-carbon energy source compared to their fossil fuel counterparts.
- If your primary focus is carbon sequestration: The creation of stable bio-char makes pyrolysis one of the most promising technologies for actively removing atmospheric CO₂ and locking it away for the long term.
Ultimately, pyrolysis emissions must be judged by their net effect on the environment, not in isolation.
Summary Table:
| Gas Type | Primary Components | Key Characteristics |
|---|---|---|
| Primary Product (Syngas) | Hydrogen (H₂), Carbon Monoxide (CO) | Combustible fuel for energy or chemical feedstock. |
| Secondary Byproducts | Carbon Dioxide (CO₂), Methane (CH₄), Nitrogen Compounds, Water Vapor, Tar | Unavoidable emissions requiring proper management. |
Ready to harness the power of pyrolysis for waste management, energy production, or carbon sequestration? KINTEK specializes in advanced laboratory equipment and consumables to support your research and development in pyrolysis technology. Whether you are optimizing process control, analyzing feedstock, or evaluating emissions, our solutions are designed to enhance your lab's efficiency and accuracy. Contact us today to discover how KINTEK can be your partner in sustainable innovation!
Visual Guide
Related Products
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the disadvantages of sputtering process? Key Limitations in Thin-Film Deposition
- How thick is the sputter coating for SEM? Achieve Optimal Imaging & Analysis
- What is the laboratory apparatus for mixing? Choose the Right Tool for Your Sample Volume and Viscosity
- How does a high-precision temperature control heating system ensure accurate corrosion kinetics? Expert Lab Solutions
- Is XRF analyzer radiation safe? Learn How Modern XRF Technology Ensures Operator Safety
- What are the safety precautions for heat experiment? Essential Steps to Prevent Lab Burns and Accidents
- How does the sintering process work? A Guide to Transforming Powder into Solid Parts
- What is alloy analysis? Ensure Material Integrity and Quality Assurance



















