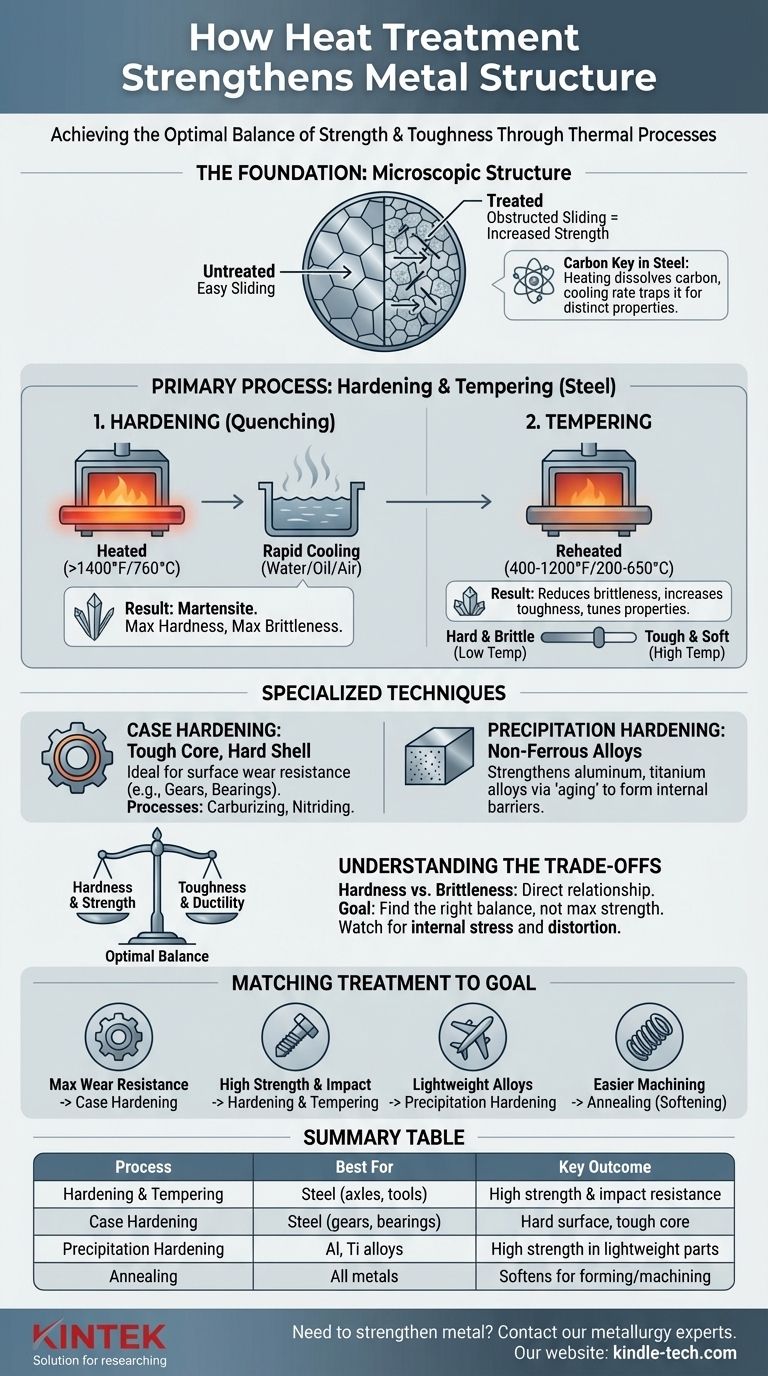
The primary heat treatment for strengthening many common metals, particularly steel, is a two-step process called hardening and tempering. This involves heating the metal to a high temperature and cooling it rapidly (quenching) to create a very hard structure, then reheating it to a lower temperature (tempering) to reduce brittleness and achieve a functional balance of strength and toughness. Other specialized methods, such as case hardening and precipitation hardening, are used for specific materials and applications.
The core principle to understand is that "strengthening" in metallurgy is never free. Heat treatments that increase a metal's hardness and tensile strength almost invariably reduce its toughness, making it more brittle. The goal is not to achieve maximum strength, but to achieve the optimal balance of properties required for the component's intended function.

The Foundation: Why Heat Changes Metal
To understand how heat treatments work, you must first understand the microscopic structure of metals. They are not uniform solids but are composed of individual crystals, or "grains."
The Role of Crystal Structure
Metals bend and deform when planes of atoms within these grains slide past one another. Strengthening a metal involves introducing obstacles that make this sliding motion more difficult.
Heat treatments accomplish this by changing the size, shape, and composition of these internal grains.
The Carbon Key in Steel
For steel, the most common structural metal, the key ingredient is carbon. While steel is mostly iron, the small amount of carbon present allows for dramatic transformations when heated and cooled.
Different cooling rates trap carbon in different crystal structures, each with unique properties. Fast cooling traps it in a hard, brittle structure, while slow cooling allows it to form a soft, ductile structure.
Primary Strengthening Processes for Steel
For most structural steels, strengthening is achieved through a controlled sequence of quenching and tempering.
Step 1: Hardening (Quenching)
Hardening is the process of creating maximum hardness. The metal is heated to a critical temperature (typically above 1,400°F or 760°C) where its crystal structure changes into a phase called austenite, which can dissolve carbon.
The metal is then rapidly cooled, or quenched, in a medium like water, oil, or air. This sudden drop in temperature traps the carbon atoms in a highly strained, needle-like crystal structure called martensite. Martensite is extremely hard and strong but also very brittle.
Step 2: Tempering
A part made only of martensite is often too brittle for practical use; it would shatter like glass under impact. Tempering is the crucial follow-up step to reduce this brittleness.
The hardened part is reheated to a much lower temperature (e.g., 400-1,200°F or 200-650°C) and held for a set time. This process relieves internal stresses and allows some of the martensite to transform into a more stable structure, significantly increasing the metal's toughness and ductility.
The final properties are "tuned" by the tempering temperature. A lower temperature results in higher hardness but less toughness, while a higher temperature creates a tougher but softer part.
Specialized Strengthening Techniques
Not all strengthening involves hardening the entire part. Specialized methods target either the surface of a component or are designed for non-steel alloys.
Case Hardening: A Tough Core, A Hard Shell
Case hardening creates a hard, wear-resistant surface (the "case") while maintaining a softer, tougher interior (the "core"). This is ideal for parts like gears and bearings that need to resist surface wear while also withstanding impact loads without shattering.
Processes like carburizing (adding carbon to the surface) or nitriding (adding nitrogen) are used to enrich the surface chemistry before a final heat treatment, creating a component with the best of both worlds.
Precipitation Hardening: For Non-Ferrous Alloys
This method, also known as age hardening, is the primary way to strengthen many aluminum, titanium, and nickel-based alloys.
The process involves heating the alloy to dissolve alloying elements into a solid solution, quenching it to lock them in place, and then "aging" it at a low temperature. During aging, tiny, hard particles (precipitates) form within the metal's structure. These particles act as microscopic barriers that obstruct deformation and dramatically increase strength.
Understanding the Trade-offs: Strength vs. Toughness
Selecting a heat treatment requires a clear understanding of its consequences. Chasing a single property, like maximum hardness, almost always leads to failure.
The Hardness vs. Brittleness Curve
There is a direct and unavoidable relationship between hardness and brittleness. As a material gets harder, it loses its ability to deform or absorb energy before fracturing.
Think of the difference between a steel paperclip and a glass rod. The paperclip (soft and tough) can bend significantly before it breaks. The glass rod (hard and brittle) can withstand a high load but will shatter with almost no warning or bending. Tempering allows you to choose a precise point on this spectrum.
Internal Stress and Distortion
The rapid cooling involved in quenching introduces significant internal stress into a metal part. This stress can cause the part to warp, distort, or even crack, especially in components with complex geometries or sharp corners.
Processes like tempering are essential for relieving these stresses. In some cases, slower quenching media (like oil instead of water) or techniques that don't require a quench (like nitriding) are chosen specifically to minimize distortion.
Why "Stronger" Isn't Always Better
A screwdriver tip that is hardened to its maximum potential might be so brittle that it shatters the first time it encounters a stubborn screw. A slightly softer, tougher tip would be far more durable.
Similarly, an axle that is excessively hard would be prone to catastrophic failure from a single pothole impact. The correct choice is a treatment that provides enough toughness to absorb such shocks safely.
Matching the Treatment to Your Goal
The right process depends entirely on the material you are using and the demands of the final application.
- If your primary focus is maximum wear resistance and surface hardness: Case hardening (like carburizing or nitriding) is your best approach for steel parts like gears or camshafts.
- If your primary focus is high overall strength combined with impact resistance: Through-hardening (quenching) followed by tempering is the standard for components like axles, bolts, and structural tools.
- If your primary focus is strengthening lightweight alloys like aluminum: Precipitation hardening is the correct and only effective process.
- If your primary focus is making a metal easier to machine or form: You need a softening process like annealing, which reverses the effects of hardening.
Ultimately, choosing a heat treatment is a deliberate engineering decision based on a clear understanding of the final application's requirements.
Summary Table:
| Strengthening Process | Best For | Key Outcome |
|---|---|---|
| Hardening & Tempering | Steel (axles, tools) | High strength & impact resistance |
| Case Hardening | Steel (gears, bearings) | Hard surface, tough core |
| Precipitation Hardening | Aluminum, titanium alloys | High strength in lightweight parts |
| Annealing | All metals (pre-machining) | Softens for easier forming/machining |
Need to strengthen a metal component for your project? KINTEK specializes in lab equipment and consumables for material testing and heat treatment processes. Whether you're working with steel, aluminum, or other alloys, our expertise can help you achieve the optimal balance of strength, toughness, and durability. Contact our metallurgy experts today to discuss your specific requirements and find the right solution for your laboratory or production needs.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost
- What is a vacuum furnace? The Ultimate Guide to Contamination-Free Thermal Processing
- What is vacuum furnace high temperature? Unlock the Range for Your Material Processing
- What are vacuum furnace parts? A Guide to the Core Systems for Precision Heat Treatment
- What are vacuum furnaces used for? Unlock Ultimate Material Purity and Performance



















