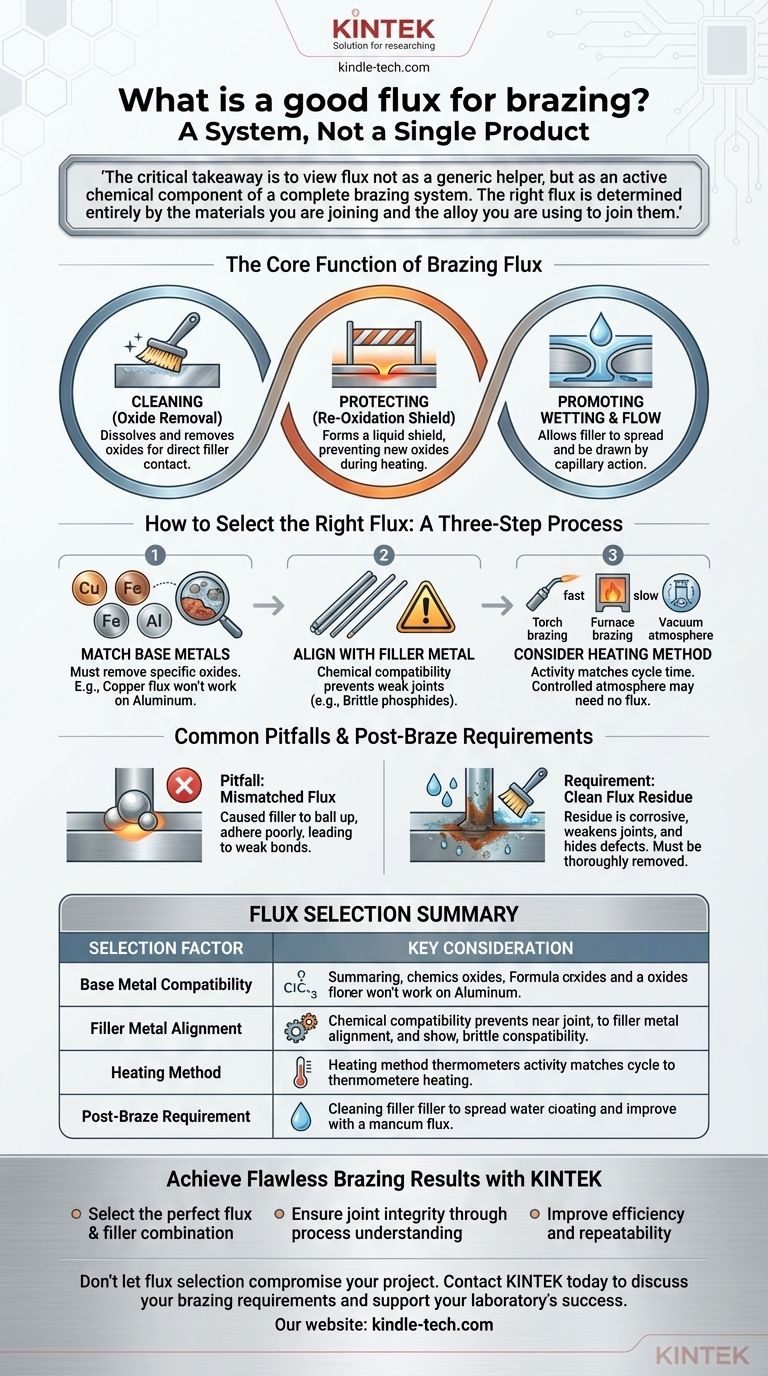
A good flux for brazing is not a single product, but rather a chemical agent specifically chosen to be compatible with your base metals, filler metal, and heating process. Its primary job is to chemically clean and protect the metal surfaces during heating, ensuring the molten filler metal can form a strong, seamless bond.
The critical takeaway is to view flux not as a generic helper, but as an active chemical component of a complete brazing system. The right flux is determined entirely by the materials you are joining and the alloy you are using to join them.

The Core Function of Brazing Flux
To select the right flux, you must first understand its three essential roles in the brazing process. Each function is critical for achieving a sound joint.
Cleaning the Surface of Oxides
All metals, when exposed to air, develop a thin, invisible layer of oxides. This layer acts as a barrier, preventing the molten brazing alloy from making direct contact with the pure base metal beneath it.
Flux is designed to dissolve and remove these oxides when heated, exposing a chemically clean surface ready for bonding.
Protecting Against Re-Oxidation
As you heat the parts to brazing temperature, the rate of oxidation increases dramatically. The flux melts and spreads over the joint area, forming a protective liquid shield.
This shield blocks oxygen from the atmosphere, preventing new, stubborn oxides from forming on the clean surfaces during the heating cycle.
Promoting Wetting and Flow
With the surfaces clean and protected, the molten filler metal can now "wet" the base metals. This means it can spread evenly across the surfaces, much like water on clean glass.
This wetting action allows capillary action to draw the filler metal deep into the tight-fitting joint, ensuring a complete and robust bond.
How to Select the Right Flux
Choosing a flux is a process of matching its chemical properties to the specific demands of your application. There is no universal solution.
Match the Flux to Your Base Metals
The single most important factor is chemical compatibility. The flux must be formulated to aggressively remove the specific type of oxide formed by your base metals.
A flux designed for copper alloys will be ineffective on stainless steel, and one for aluminum will not work on iron. Using an incorrect flux will fail to clean the surface, resulting in a failed joint.
Align with Your Filler Metal
The flux must also be chemically compatible with the filler metal. An improper combination can lead to undesirable chemical reactions.
For example, using certain phosphorus-bearing filler alloys on iron or nickel can create brittle compounds called phosphides, severely weakening the joint. The flux and filler must work together seamlessly.
Consider Your Heating Method and Cycle Time
The activity level of a flux is designed for a specific temperature range and time. A fast, high-temperature process like torch brazing requires a highly active flux that can work quickly.
Conversely, a slower process like furnace brazing often uses a less active, longer-lasting flux. In some cases, such as vacuum or controlled-atmosphere furnace brazing, the protective atmosphere removes oxides, eliminating the need for flux entirely.
Common Pitfalls and Post-Braze Requirements
Understanding what can go wrong is just as important as knowing the ideal process. Awareness of these issues is a hallmark of professional work.
The Danger of Mismatched Flux
Using the wrong flux is a primary cause of brazing failure. The symptoms are clear: the filler metal will ball up and refuse to flow, or it will adhere poorly, creating a weak and unreliable bond.
The Corrosive Nature of Flux Residue
Most brazing fluxes are chemically aggressive by design. If residue is left on the part after brazing, it can absorb moisture from the air and cause severe corrosion over time.
This corrosion can weaken the joint and damage the surrounding base metal. Therefore, all excess flux must be thoroughly removed after the part has cooled.
Forgetting Post-Braze Cleaning
Failing to remove flux residue not only risks corrosion but also hinders visual inspection of the joint. A layer of hardened flux can easily hide cracks, voids, or areas of non-adhesion.
It also interferes with any subsequent finishing operations like painting, plating, or powder coating.
Making the Right Choice for Your Project
Your choice of flux must be a deliberate decision based on the fundamental components of your brazing operation.
- If your primary focus is joining common metals like copper or steel: Select a general-purpose flux that is explicitly listed as compatible with your chosen base metal and filler alloy family.
- If your primary focus is joining specialty alloys or dissimilar metals: Your decision must be driven by precise chemical compatibility; consult the filler metal manufacturer's technical data sheets for specific flux recommendations.
- If your primary focus is high-volume production or sensitive electronics: Investigate controlled-atmosphere furnace brazing to eliminate the need for flux and the associated risk of corrosive residue.
Ultimately, successful brazing depends on treating the base metal, filler metal, and flux as an interconnected system.
Summary Table:
| Selection Factor | Key Consideration |
|---|---|
| Base Metal Compatibility | Flux must be formulated to remove the specific oxides on your metals (e.g., copper, stainless steel, aluminum). |
| Filler Metal Alignment | Must be chemically compatible to prevent undesirable reactions that weaken the joint. |
| Heating Method | Torch brazing needs a fast-acting flux; furnace brazing may use a longer-lasting type or none at all in a controlled atmosphere. |
| Post-Braze Requirement | Most fluxes leave corrosive residue that must be thoroughly cleaned after the part cools. |
Achieve Flawless Brazing Results with KINTEK
Choosing the correct flux is critical, but it's just one part of a successful brazing system. KINTEK specializes in lab equipment and consumables, providing the high-quality materials and expert support your laboratory needs for precise and reliable metal joining.
We help you:
- Select the perfect flux and filler metal combination for your specific base metals and application.
- Ensure joint integrity by understanding the complete brazing process, from surface preparation to post-braze cleaning.
- Improve efficiency and repeatability in your research or production workflows.
Don't let flux selection compromise your project. Let our expertise guide you to stronger, more reliable bonds.
Contact KINTEK today to discuss your brazing requirements and discover how we can support your laboratory's success.
Visual Guide
Related Products
- Conductive Carbon Fiber Brush for Static Removal and Cleaning
- Warm Isostatic Press for Solid State Battery Research
- Custom PTFE Teflon Parts Manufacturer for Hollow Etching Flower Basket ITO FTO Developing Glue Removal
- Laboratory Hydraulic Press Lab Pellet Press Machine for Glove Box
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- What is the process of sputtering target? A Step-by-Step Guide to Thin Film Deposition
- How is hot forging different from cold forging? Key Differences in Strength, Cost & Applications
- What are the effects of pyrolysis on biomass? A Tunable Process for Biochar, Bio-oil, and Syngas
- How does biochar affect crop yield? A Guide to Maximizing Soil Health and Long-Term Productivity
- What are the disadvantages of pressing and sintering? Understand the Trade-offs in Powder Metallurgy
- What is the injection molding process? A Guide to High-Volume Part Production
- Why is ultrasonic treatment essential for PAAMP-b-PVK synthesis? Achieve Surfactant-Free Emulsion Polymerization
- What is the evaporation method of extraction? A Guide to Solvent Removal & Thin Film Coating



















