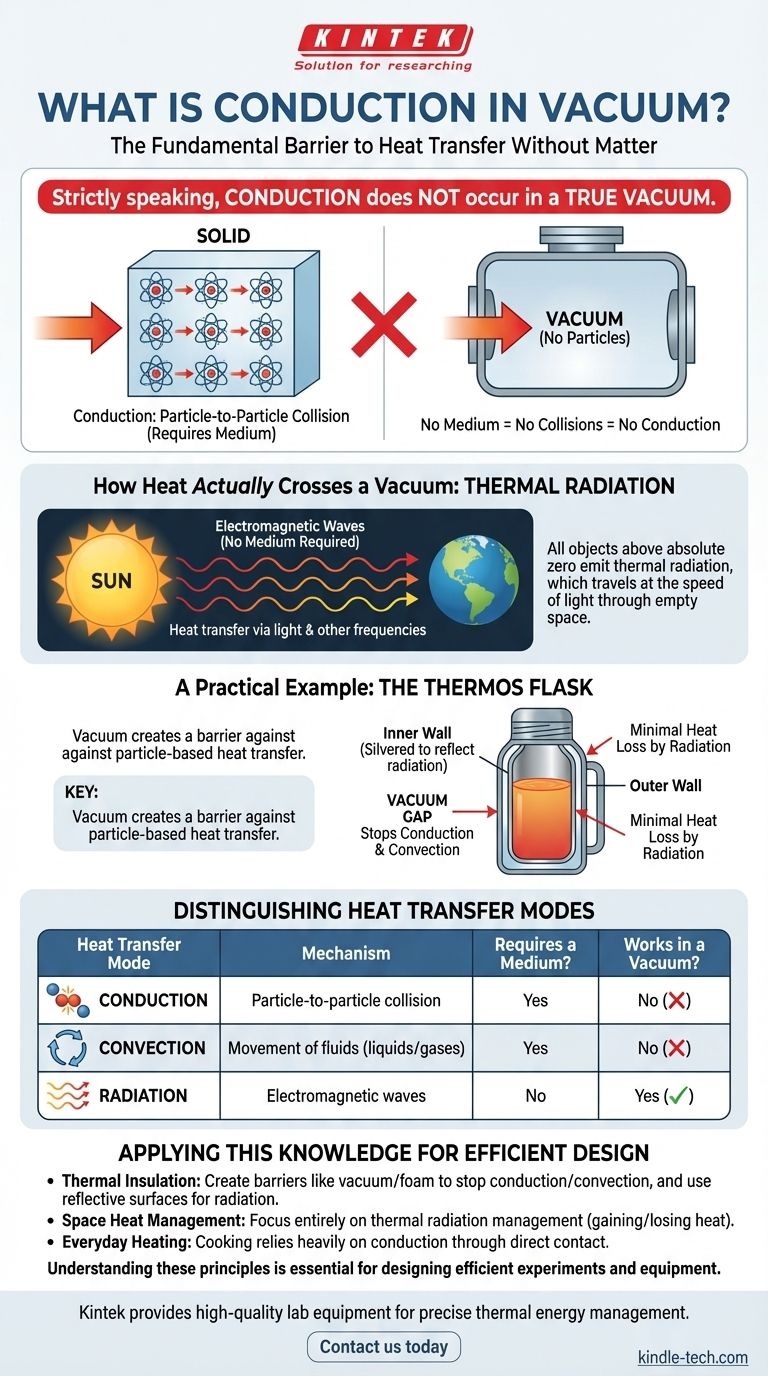
Strictly speaking, conduction in a true vacuum does not occur. Conduction is the transfer of heat through direct molecular collision, a process that requires a physical medium. Because a vacuum is an absence of matter, there are no particles to collide and transfer thermal energy, making conduction impossible.
The core misunderstanding arises from applying a concept (conduction) to an environment (a vacuum) that fundamentally lacks the necessary components for it to happen. Heat can cross a vacuum, but it does so exclusively through the mechanism of thermal radiation, not conduction or convection.

The Fundamental Barrier to Conduction
To understand why conduction fails in a vacuum, we must first define how it works. It is a process of direct, particle-to-particle energy transfer.
Conduction is a Chain Reaction
Think of heat as the vibration of atoms and molecules. In a solid, when one side of an object is heated, its atoms vibrate more intensely.
These energetic atoms then jostle and collide with their immediate neighbors, transferring that vibrational energy. This process continues down the line, like a series of falling dominoes, until the heat has propagated through the material.
A Vacuum Has No Particles
A vacuum, by its very definition, is a space devoid of matter. There are no atoms or molecules to form the "chain" needed for conduction.
Without a medium, there can be no particle-to-particle collisions. The pathway for conduction is completely broken.
How Heat Actually Crosses a Vacuum
If conduction is impossible, how does the Sun's heat reach Earth? The answer is an entirely different mechanism that requires no medium at all.
The Role of Thermal Radiation
Every object with a temperature above absolute zero (0 Kelvin) emits its thermal energy as electromagnetic waves. This is called thermal radiation.
These waves, which include infrared light, visible light, and other frequencies, travel at the speed of light and can move through the empty void of space.
No Medium Required
Unlike conduction or convection, thermal radiation does not need any particles to propagate. When these electromagnetic waves strike an object, their energy is absorbed, causing the object's atoms to vibrate more intensely—which we perceive as an increase in heat.
A Practical Example: The Thermos Flask
A thermos (or vacuum flask) is a perfect real-world application of this principle. It is designed with a gap between its inner and outer walls from which the air has been removed, creating a vacuum.
This vacuum acts as a powerful insulator precisely because it stops heat transfer by both conduction and convection. Heat cannot conduct across the empty space. The silvered surfaces of the inner walls also serve to minimize heat transfer by radiation.
Distinguishing the Three Modes of Heat Transfer
Confusion often arises from mixing up the three distinct ways heat can move. Understanding each one's requirement clarifies why only one works in a vacuum.
Conduction: Direct Contact
This is heat transfer through a substance via direct molecular contact. It is most effective in solids, like a metal spoon heating up in a hot cup of tea. It requires a medium.
Convection: Fluid Movement
This is heat transfer through the movement of fluids (liquids or gases). Warmer, less dense fluid rises, and cooler, denser fluid sinks, creating a current that circulates heat. Think of boiling water or a room heater. It requires a fluid medium.
Radiation: Electromagnetic Waves
This is heat transfer via electromagnetic waves. It is the only mode of heat transfer that does not require a medium and can therefore operate across the vacuum of space.
Applying This Knowledge
Understanding these distinctions is critical for solving practical engineering and design problems.
- If your primary focus is thermal insulation (like a thermos or home insulation): Your goal is to create barriers that stop conduction and convection (like a vacuum or foam) and use surfaces that minimize radiation (like reflective foil).
- If your primary focus is heat management in space (like a satellite): You must focus entirely on managing thermal radiation, as it's the only way your object can gain heat from the sun or lose its own heat into deep space.
- If your primary focus is cooking on a stovetop: You are primarily using conduction from the burner to the pan, and subsequently into the food through direct contact.
Recognizing which heat transfer mechanism dominates a given environment is the first step toward effectively controlling it.
Summary Table:
| Heat Transfer Mode | Mechanism | Requires a Medium? | Works in a Vacuum? |
|---|---|---|---|
| Conduction | Particle-to-particle collision | Yes | No |
| Convection | Movement of fluids (liquids/gases) | Yes | No |
| Radiation | Electromagnetic waves | No | Yes |
Need to control heat transfer in your lab processes? Understanding the principles of conduction, convection, and radiation is essential for designing efficient experiments and equipment. KINTEK specializes in providing high-quality lab equipment and consumables that help you manage thermal energy effectively. Whether you're working with vacuum furnaces, insulation, or thermal analysis, our solutions are designed to meet the precise needs of your laboratory. Contact us today to learn how we can support your research and innovation!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- What kind of material is full annealing applied to? Optimize Steel for Maximum Machinability
- How is heat transfer in liquids different from that in a vacuum? Mastering Thermal Management for Your Lab
- When would you use tempering? Optimize Steel Hardness vs. Toughness for Your Application
- What is a vacuum brazing furnace? Achieve Flawless, High-Strength Joints for Critical Applications
- What is the temperature used in hardening? Master the Key to Steel Hardening Success
- What is the temperature of a vacuum brazing furnace? Key metrics for precision joining
- What is the primary function of a high vacuum furnace in brazing? Achieve Flux-Free, High-Strength Stainless Steel Joints
- What is the primary function of a vacuum brazing furnace for Niobium Permeators? Achieve High-Integrity Hermetic Seals



















