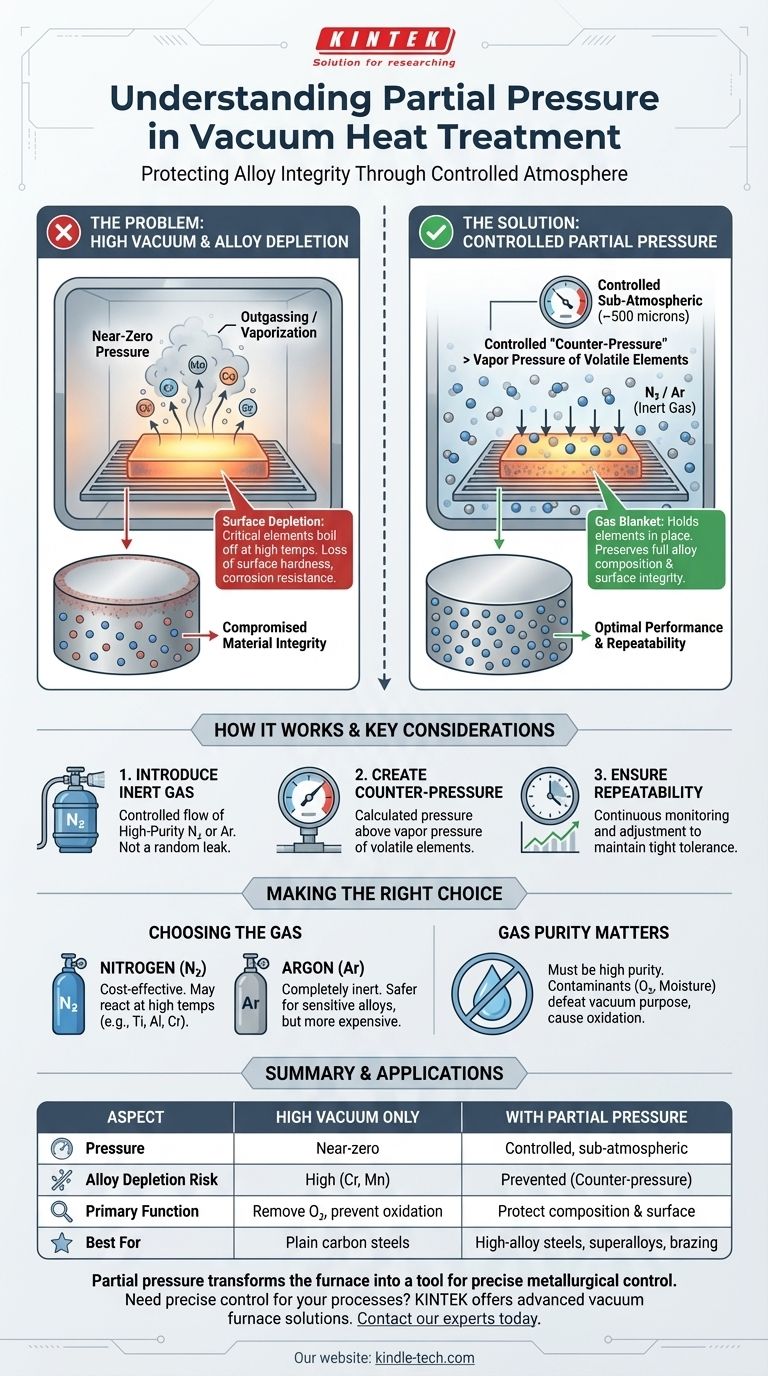
In vacuum heat treatment, partial pressure is the deliberate introduction of a specific, inert gas (like nitrogen or argon) into the furnace chamber. This process raises the pressure from a deep vacuum to a controlled, sub-atmospheric level, typically around 500 microns. The purpose is not to create a "weaker" vacuum, but to establish a precise, protective atmosphere.
The core function of partial pressure is to create a controlled "counter-pressure" on the surface of the workpiece. This counter-pressure is strategically set to be higher than the vapor pressure of volatile alloying elements in the metal, preventing them from boiling off or sublimating at high temperatures.

The Problem: High Vacuum Isn't Always Better
While a high vacuum is excellent for removing oxygen and preventing oxidation, it creates another problem when processing certain materials at elevated temperatures. The near-zero pressure environment can cause critical elements within the metal alloy to vaporize directly from the surface.
Understanding Vapor Pressure
Every element has a vapor pressure, which is its tendency to turn into a gas at a given temperature. In a high vacuum, there is virtually no external pressure pushing down on the material's surface.
As the furnace temperature rises, the vapor pressure of certain alloying elements (like chromium, manganese, or copper) can exceed the extremely low pressure of the surrounding vacuum.
The Risk of Alloy Depletion
When an element's vapor pressure surpasses the furnace pressure, it begins to sublimate—turning from a solid directly into a gas. This process is often called "outgassing" or "vaporization."
This effect strips these critical elements from the surface of the part, leaving behind a depleted layer that no longer has the same chemical composition as the core material.
The Consequences for Material Integrity
This surface depletion can be catastrophic for the component's performance. It can lead to a loss of surface hardness, reduced corrosion resistance, and compromised mechanical properties. The part may meet core hardness specifications but fail prematurely in service due to its weakened surface.
How Partial Pressure Solves the Problem
Partial pressure is the elegant engineering solution to prevent alloy depletion. It works by fundamentally changing the pressure dynamics inside the furnace.
Introducing a Controlled Gas
The process involves backfilling the high-vacuum chamber with a precise amount of a high-purity inert gas, most commonly nitrogen or argon.
This is not a random leak; it is a highly controlled flow managed by the furnace's control system to maintain a specific pressure setpoint.
Creating a Protective Counter-Pressure
The introduced gas molecules create a positive pressure on the workpiece. This pressure is carefully calculated to be above the vapor pressure of the volatile elements at the target process temperature.
This "gas blanket" effectively holds the alloying elements in place, preventing them from escaping the material's surface even at extreme temperatures.
Ensuring Process Repeatability
Modern vacuum furnaces continuously monitor and adjust the gas flow to maintain the partial pressure within a very tight tolerance.
As noted in control logic, if the pressure deviates from the setpoint, the heating program is automatically paused until the correct atmosphere is restored. This guarantees that every part in the load is treated under the exact same, optimal conditions.
Understanding the Key Considerations
Using partial pressure is a precision technique, and making the right choices is critical for success.
Choosing the Right Gas
The choice between nitrogen and argon is not arbitrary. Nitrogen is cost-effective but can react with certain elements at high temperatures (like titanium, aluminum, and chromium), potentially forming unwanted nitrides on the surface.
Argon is completely inert and will not react with the workpiece, making it the safer choice for sensitive alloys, although it is more expensive.
The Need for High Purity
The backfill gas must be extremely pure. Any contaminants in the gas, such as oxygen or moisture, will be introduced directly into the heating chamber, defeating the purpose of the vacuum process and leading to oxidation or discoloration.
Making the Right Choice for Your Process
Applying partial pressure correctly depends entirely on the material being treated and the desired outcome.
- If your primary focus is treating high-alloy tool steels, stainless steels, or superalloys: Partial pressure is essential to prevent the vaporization of chromium and other key elements, ensuring full surface hardness and corrosion resistance.
- If your primary focus is vacuum brazing: Partial pressure is critical for preventing volatile elements within the braze filler metal (like zinc or cadmium) from boiling off before the alloy reaches its melting point.
- If your primary focus is processing plain carbon steels or alloys with no volatile elements: A standard high-vacuum process is likely sufficient and may be more efficient, as there is no risk of alloy depletion.
Ultimately, partial pressure transforms the vacuum furnace from a simple heating chamber into a tool for precise metallurgical control.
Summary Table:
| Aspect | High Vacuum Only | With Partial Pressure |
|---|---|---|
| Atmosphere Control | Near-zero pressure | Controlled, sub-atmospheric pressure (e.g., ~500 microns) |
| Risk of Alloy Depletion | High for volatile elements (Cr, Mn) | Prevented by counter-pressure |
| Primary Function | Remove oxygen, prevent oxidation | Protect alloy composition & surface integrity |
| Ideal Gas Used | Not applicable | Nitrogen (cost-effective) or Argon (inert) |
| Best For | Plain carbon steels | High-alloy steels, superalloys, vacuum brazing |
Need precise metallurgical control for your heat treatment processes?
KINTEK specializes in advanced lab equipment and consumables, including vacuum furnace solutions designed for reliable partial pressure control. Our expertise ensures your high-value components maintain their surface integrity and mechanical properties.
Contact our experts today to discuss how our solutions can enhance your laboratory's capabilities and protect your critical materials.
Visual Guide
Related Products
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What is the effect of high-temperature vacuum on passivation films? Master Refractory Alloy Stability
- Why is graphite used in furnaces? For Extreme Heat, Purity, and Efficiency
- What is the difference between hardening quenching and tempering? A Guide to the Complete Heat Treatment Process
- What is the mechanism of using pure titanium granules as a getter? Enhancing High-Entropy Alloy Purity
- What is the function of calciner? Unlock Material Transformation for Your Industry
- What are the advantages of vacuum hardening? Achieve Superior Precision and Cleanliness for Critical Components
- Why is a vacuum drying system utilized for PDVB nanoparticle preparation? Preserve Structure and Chemical Activity
- How does a high-temperature laboratory furnace modify Li–Al LDH during catalyst pretreatment? Enhance Catalytic Activity



















