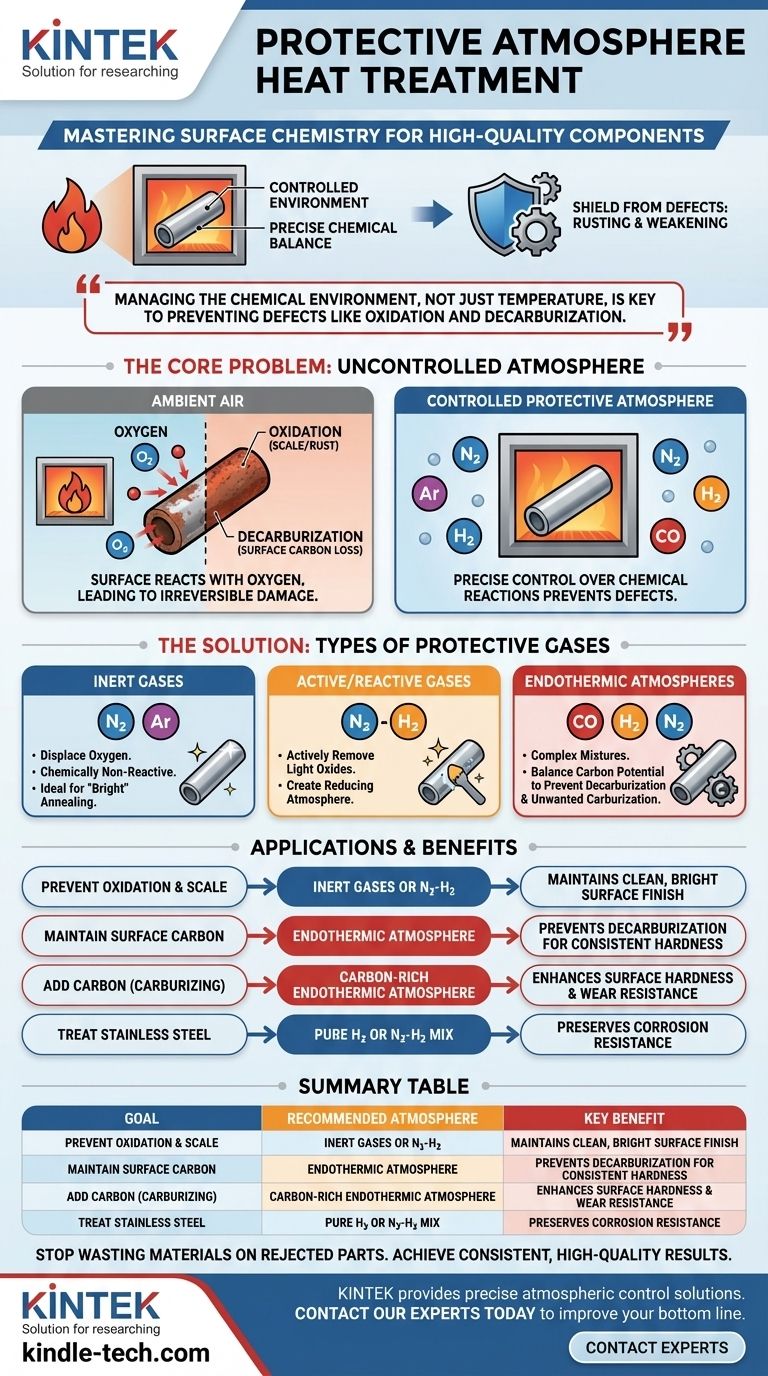
At its core, protective atmosphere heat treatment is a highly controlled industrial process where a material, typically metal, is heated and cooled within a specific, engineered gas environment. The purpose of this atmosphere is to shield the material from harmful chemical reactions—like rusting or weakening—that would otherwise occur when heated in ambient air.
The central challenge in heat treatment isn't just managing temperature; it's managing the chemical environment. A protective atmosphere gives you precise control over the surface chemistry of a part, preventing defects like oxidation and decarburization to ensure the final product meets its required quality and performance standards.

The Core Problem: What Happens in an Uncontrolled Atmosphere
When metals are heated to high temperatures in normal air, their surfaces react with the surrounding gases, primarily oxygen. This leads to irreversible and often detrimental changes.
The Impact of Oxidation
Oxidation is the chemical reaction between the hot metal surface and oxygen. For steels, this is commonly seen as the formation of scale or rust.
This surface scale is not just a cosmetic issue. It can alter the dimensions of a part, compromise its strength, and negatively impact its performance in its final application.
The Challenge of Decarburization
Decarburization is a specific problem for steel. It is the process where carbon atoms diffuse out from the surface of the steel, a reaction often driven by oxygen and water vapor at high temperatures.
Since carbon is the primary element that gives steel its hardness and strength, losing it from the surface makes the part softer and less resistant to wear. This can lead to premature failure.
The Business Consequences of Surface Defects
Failing to control the furnace atmosphere can lead to parts that do not meet specifications. This results in rejected batches, wasted materials, decreased profitability, and significant risks if a defective part enters the supply chain.
How Protective Atmospheres Provide the Solution
A protective atmosphere replaces the ambient air in a furnace with a carefully managed gas or mixture of gases. This allows for precise control over the chemical reactions at the metal's surface.
The Principle of Atmospheric Control
The goal is to create an environment that is either chemically non-reactive (inert) or actively balanced to the material being treated. By controlling the levels of gases like oxygen, carbon monoxide, and water vapor, engineers can dictate the final surface condition of the workpiece.
Common Types of Protective Gases
Protective atmospheres range from simple to complex, depending on the desired outcome.
- Inert Gases: Gases like Argon (Ar) and Nitrogen (N2) are used to simply displace oxygen. They are chemically non-reactive and prevent oxidation from occurring, which is ideal for "bright" annealing or hardening where the surface must remain clean.
- Active (or Reactive) Gases: These are mixtures that play an active role. For example, a nitrogen-hydrogen (N2-H2) blend can create a "reducing" atmosphere that not only prevents oxidation but can also remove existing light oxides.
- Endothermic Atmospheres: These complex mixtures (often CO-H2-N2) are generated to have a specific "carbon potential." They are meticulously balanced to prevent both decarburization and unwanted carburization (the addition of carbon), making them essential for treating high-carbon steels.
Understanding the Trade-offs and Applications
Using a protective atmosphere adds complexity and cost to the heat treatment process, but this is a necessary trade-off for achieving high-quality, reliable components.
Matching the Atmosphere to the Material
The choice of atmosphere is critical. An atmosphere suitable for a low-alloy structural steel may be completely wrong for stainless steel. For example, endothermic gas is widely used for carburizing and neutral hardening, while stainless steel often requires a pure hydrogen or nitrogen-hydrogen atmosphere to maintain its corrosion resistance.
The Cost of Precision
Generating and maintaining these atmospheres requires specialized equipment, including gas generators, mixers, and sophisticated control systems. While this represents a significant investment, it is often far less than the cost of producing an entire batch of rejected parts.
Making the Right Choice for Your Goal
Selecting the correct protective atmosphere depends entirely on the material being treated and the desired outcome.
- If your primary focus is preventing surface rust and scale (oxidation): An inert gas like nitrogen or a nitrogen-hydrogen blend is often the most effective and economical choice.
- If your primary focus is maintaining the exact surface carbon of a steel part: You require a precisely controlled endothermic atmosphere with a carbon potential matched to your material.
- If your primary focus is adding carbon to the surface (carburizing or carbonitriding): A specialized, carbon-rich endothermic atmosphere is non-negotiable to ensure the process is consistent and effective.
Ultimately, controlling the furnace atmosphere gives you direct control over the final quality, reliability, and performance of your heat-treated components.
Summary Table:
| Goal | Recommended Atmosphere | Key Benefit |
|---|---|---|
| Prevent Oxidation & Scale | Inert Gases (Nitrogen, Argon) or N2-H2 Blend | Maintains clean, bright surface finish |
| Maintain Surface Carbon Content | Endothermic Atmosphere (CO-H2-N2) | Prevents decarburization for consistent hardness |
| Add Carbon to Surface (Carburizing) | Carbon-Rich Endothermic Atmosphere | Enhances surface hardness and wear resistance |
| Treat Stainless Steel | Pure Hydrogen or Nitrogen-Hydrogen Mix | Preserves corrosion resistance properties |
Stop wasting materials on rejected parts. Protect your heat-treated components from costly surface defects like oxidation and decarburization. KINTEK specializes in lab equipment and consumables, providing the precise atmospheric control solutions your laboratory needs. Contact our experts today to discuss how we can help you achieve consistent, high-quality results and improve your bottom line.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Vertical Laboratory Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Multi-zone Laboratory Tube Furnace
People Also Ask
- What gases are used in inert atmospheres? Choose the Right Gas for Non-Reactive Environments
- What provides an inert atmosphere? Achieve Safety and Purity with Nitrogen, Argon, or CO2
- What is an inert condition? A Guide to Preventing Fires and Explosions
- Why nitrogen is used in annealing furnace? To prevent oxidation and decarburization for superior metal quality
- How do you make an inert atmosphere? Master Safe, Pure Processes with Inerting



















