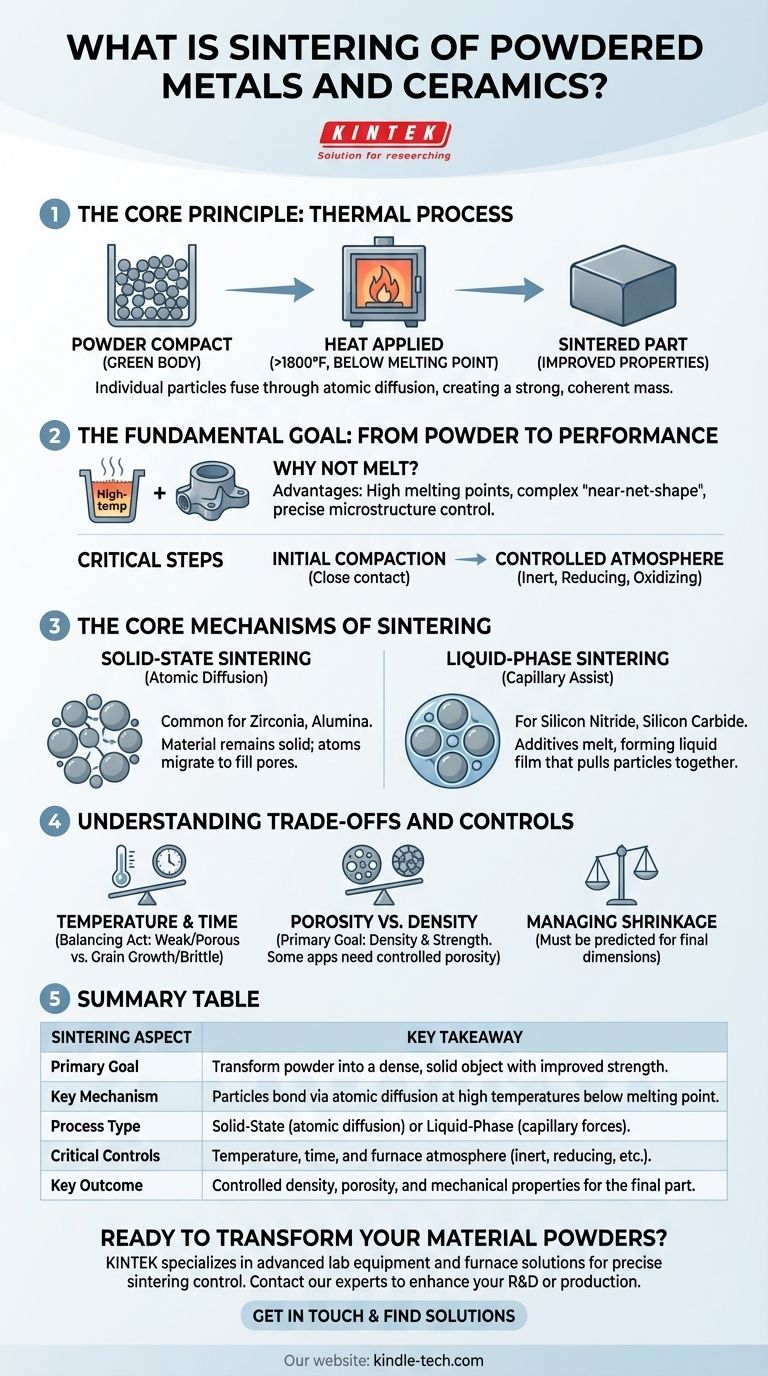
In essence, sintering is a thermal process used to convert a collection of metal or ceramic powder into a solid, dense object. It achieves this by heating the material to a high temperature, typically above 1800°F, but below its full melting point. At this temperature, the individual particles fuse together through atomic diffusion, creating a strong, coherent mass with significantly improved mechanical properties.
The core principle of sintering is not to melt the material, but to use controlled heat to encourage individual particles to bond and densify. This transforms a fragile powder compact into a robust, engineered component.

The Fundamental Goal: From Powder to Performance
Sintering is a critical step in powder metallurgy and ceramic processing. It is the bridge between a loosely compacted shape and a functional, high-strength part.
Why Not Simply Melt the Material?
While melting and casting can form solid parts, sintering offers distinct advantages. It allows for the creation of parts from materials with extremely high melting points and enables the production of complex, "near-net-shape" components that require minimal finishing. It also provides precise control over the final microstructure, including properties like density and porosity.
The Role of Initial Compaction
Before sintering, the powder is typically pressed into a desired shape, often called a "green body." This initial compaction is crucial as it forces the particles into close contact. The more intimate the contact, the more efficiently the atoms can diffuse between particles during the heating process.
The Importance of a Controlled Atmosphere
The sintering process is conducted in a carefully controlled furnace atmosphere. Depending on the material, this can be inert (to prevent unwanted reactions), reducing (to remove surface oxides from metal powders), or oxidizing. This control is essential for preventing contamination and ensuring the material's final chemical integrity.
The Core Mechanisms of Sintering
The bonding between particles happens through two primary mechanisms, chosen based on the material being processed.
Solid-State Sintering: Atomic Diffusion
In solid-state sintering, the material remains entirely solid throughout the process. Atoms migrate across the boundaries where particles touch, gradually filling the voids (pores) between them. This causes the particles to merge and the overall part to shrink and densify.
This method is common for ceramics like zirconia and alumina. The driving force is the reduction of surface energy—it is more energetically favorable for the particles to form one solid mass than to remain as individual grains.
Liquid-Phase Sintering: A Capillary Assist
For materials that are difficult to densify, such as silicon nitride and silicon carbide, liquid-phase sintering is used. In this process, specific additives are mixed with the primary powder.
At the sintering temperature, these additives melt and form a thin liquid film around the solid particles. This liquid pulls the particles together through capillary forces, much like how wet sand sticks together. This allows for faster particle rearrangement and more rapid densification.
Understanding the Trade-offs and Controls
Successful sintering is a balancing act. The final properties of the component are dictated by careful control over the process parameters.
Temperature and Time
The two most critical variables are temperature and time. Insufficient heat or time will result in a weak, porous part with incomplete bonding. However, excessive heat or time can cause undesirable grain growth, which can make the final material brittle.
Porosity vs. Density
The primary objective of sintering is typically to reduce porosity and increase density. A fully dense part generally possesses the highest mechanical strength and hardness. However, in some applications like filters or self-lubricating bearings, a specific level of controlled porosity is the desired outcome.
Managing Shrinkage
As the voids between particles are eliminated, the entire component shrinks. This shrinkage is significant and must be precisely predicted and accounted for in the initial design of the mold and the green body to achieve the correct final dimensions.
Making the Right Choice for Your Application
Controlling the sintering process allows you to tailor the material's microstructure for a specific performance goal.
- If your primary focus is maximum mechanical strength: You must optimize for the highest possible density, which often involves higher temperatures, longer times, or using liquid-phase sintering to fully eliminate porosity.
- If your primary focus is thermal or electrical insulation: The key is to achieve a specific microstructure and density, as these factors directly govern the material's resistance to heat or electricity flow.
- If your primary focus is producing complex, high-precision parts: Careful control of the initial powder characteristics and predicting shrinkage during the sintering cycle are your most critical variables.
Ultimately, mastering sintering is about precisely controlling heat, time, and atmosphere to transform simple powder into a high-performance engineered component.
Summary Table:
| Sintering Aspect | Key Takeaway |
|---|---|
| Primary Goal | Transform powder into a dense, solid object with improved strength. |
| Key Mechanism | Particles bond via atomic diffusion at high temperatures below melting point. |
| Process Type | Solid-State (atomic diffusion) or Liquid-Phase (capillary forces). |
| Critical Controls | Temperature, time, and furnace atmosphere (inert, reducing, etc.). |
| Key Outcome | Controlled density, porosity, and mechanical properties for the final part. |
Ready to transform your material powders into high-performance components?
The precise control of temperature, atmosphere, and time is critical for successful sintering. KINTEK specializes in the advanced lab equipment and furnace solutions needed to master this process. Whether you are working with metals, ceramics, or advanced composites, our expertise can help you achieve the exact density, strength, and microstructure your application demands.
Contact our sintering experts today to discuss how our reliable equipment can enhance your R&D or production capabilities. Get in touch via our contact form to find the perfect solution for your lab's needs.
Visual Guide
Related Products
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the function of quartz tubes and vacuum sealing systems? Secure Your High-Purity Solid Solution Synthesis
- What is the technical value of using a quartz tube reaction chamber for static corrosion testing? Achieve Precision.
- How does an industrial tube furnace ensure the required process conditions for supercritical fluid experimental devices?
- What is the role of a tube furnace in the thermal treatment of argyrodite electrolytes? Master Ionic Conductivity
- Why use quartz tubes and vacuum sealing for sulfide solid-state electrolytes? Ensure Purity & Stoichiometry



















