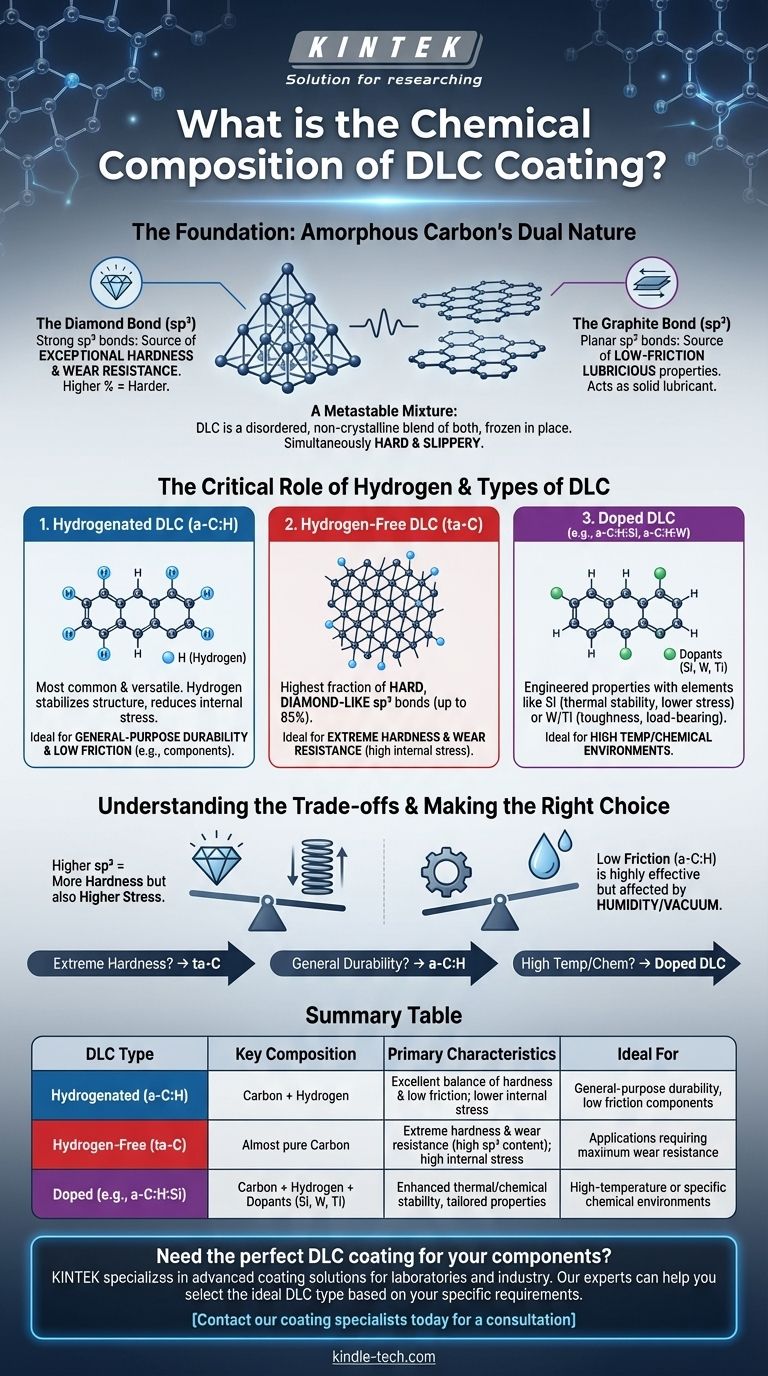
At its core, a Diamond-Like Carbon (DLC) coating is primarily composed of carbon atoms. However, unlike diamond or graphite which have a uniform crystalline structure, DLC is amorphous, meaning its atoms lack long-range order. Most commercial DLC coatings also contain a significant amount of hydrogen, which is incorporated during the deposition process from hydrocarbon source gases.
The key to understanding DLC is realizing it's not a single material, but a family of amorphous carbon coatings. Its properties are not defined simply by its elements (carbon and hydrogen), but by the ratio of diamond-like to graphite-like bonds between its carbon atoms.

The Foundation: Amorphous Carbon's Dual Nature
The unique combination of properties found in DLC—high hardness and low friction—originates from the mixture of two different types of atomic bonds that carbon can form.
The Diamond Bond (sp³)
This is the same type of bond found in natural diamond. It creates a strong, three-dimensional tetrahedral lattice.
In a DLC film, the sp³ bonds are the source of its exceptional hardness and wear resistance. A higher percentage of sp³ bonding results in a harder coating.
The Graphite Bond (sp²)
This is the bond type found in graphite. It forms planar, hexagonal sheets that are strong in their plane but slide easily over one another.
The sp² bonds are the source of DLC's low-friction, lubricious properties. These graphitic regions act as a solid lubricant on the coating's surface.
A Metastable Mixture
DLC's defining characteristic is that it is a metastable, non-crystalline mixture of both sp³ and sp² bonded carbon atoms. The manufacturing process freezes this disordered atomic structure in place, creating a material that is simultaneously hard like diamond and slippery like graphite.
The Critical Role of Hydrogen
The source hydrocarbon gas used in many deposition processes means hydrogen is often a key component of the final coating, creating what is known as hydrogenated amorphous carbon (a-C:H).
Hydrogenated DLC (a-C:H)
This is the most common and versatile form of DLC. During deposition, hydrogen atoms attach to the carbon network.
This process stabilizes the structure by terminating "dangling" bonds, which reduces internal compressive stress. This makes the coating more flexible and allows it to be applied in thicker layers without delaminating, making it ideal for a wide range of components.
Hydrogen-Free DLC (ta-C)
It is also possible to create DLC with virtually no hydrogen, known as tetrahedral amorphous carbon (ta-C).
These coatings have a much higher fraction of hard, diamond-like sp³ bonds (up to 85%). This makes them among the hardest and most wear-resistant of all DLC types, approaching the properties of pure diamond.
Understanding the Trade-offs
The specific composition and bonding structure of a DLC film create a series of performance trade-offs that are critical to understand for any application.
Hardness vs. Internal Stress
A higher sp³ content, especially in hydrogen-free (ta-C) films, yields extreme hardness. However, it also generates very high internal compressive stress, which can make the coating brittle and limit its adhesion or maximum thickness. Hydrogenated (a-C:H) films trade some hardness for lower stress and better adhesion.
Friction vs. Environment
The low friction of a-C:H coatings is highly effective, but can be influenced by the operating environment, particularly humidity. The graphitic (sp²) content that provides lubricity interacts with atmospheric moisture. In a vacuum or very dry environment, its friction coefficient can increase.
Adding Other Elements (Doping)
To further engineer properties, DLC can be "doped" with other elements. For example, adding silicon (Si) can increase thermal stability and reduce internal stress, while adding metals like tungsten (W) or titanium (Ti) can increase toughness and load-bearing capacity. This creates an even wider family of materials (e.g., a-C:H:Si or a-C:H:W).
Making the Right Choice for Your Application
Choosing the correct DLC formulation requires aligning its chemical composition and bonding with your primary engineering goal.
- If your primary focus is extreme hardness and wear resistance: A hydrogen-free (ta-C) coating with the highest possible sp³ content is the superior choice.
- If your primary focus is low friction and general-purpose durability: A hydrogenated (a-C:H) coating provides an excellent balance of lubricity, hardness, and low internal stress.
- If your primary focus is performance in high temperatures or specific chemical environments: A doped DLC (e.g., a-C:H:Si) is likely required to provide the necessary stability.
By understanding the chemical composition beyond just its elements, you can select the precise type of DLC that functions as a truly engineered surface for your component.
Summary Table:
| DLC Type | Key Composition | Primary Characteristics | Ideal For |
|---|---|---|---|
| Hydrogenated (a-C:H) | Carbon + Hydrogen | Excellent balance of hardness & low friction; lower internal stress | General-purpose durability, low friction components |
| Hydrogen-Free (ta-C) | Almost pure Carbon | Extreme hardness & wear resistance (high sp³ content); high internal stress | Applications requiring maximum wear resistance |
| Doped (e.g., a-C:H:Si) | Carbon + Hydrogen + Dopants (Si, W, Ti) | Enhanced thermal/chemical stability, tailored properties | High-temperature or specific chemical environments |
Need the perfect DLC coating for your components?
Understanding the nuances between hydrogenated (a-C:H), hydrogen-free (ta-C), and doped DLC coatings is key to achieving optimal performance. The right formulation can drastically improve hardness, reduce friction, and extend the life of your parts.
KINTEK specializes in advanced coating solutions for laboratories and industry. Our experts can help you select the ideal DLC type based on your specific requirements for wear resistance, lubricity, and environmental stability.
Contact our coating specialists today for a consultation and see how our engineered surfaces can solve your toughest application challenges.
Visual Guide
Related Products
- Custom CVD Diamond Coating for Lab Applications
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Electrolytic Electrochemical Cell for Coating Evaluation
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Precision Wire Saw Laboratory Cutting Machine with 800mm x 800mm Workbench for Diamond Single Wire Circular Small Cutting
People Also Ask
- How long does diamond coating last? Maximize Lifespan with the Right Coating for Your Application
- What is diamond coating film? A Thin Layer of Diamond for Extreme Performance
- What are the three types of coating? A Guide to Architectural, Industrial, and Special Purpose
- What are diamond coated films? Enhance Materials with Super-Hard, Transparent Layers
- How thick is CVD diamond coating? Balancing Durability and Stress for Optimal Performance









