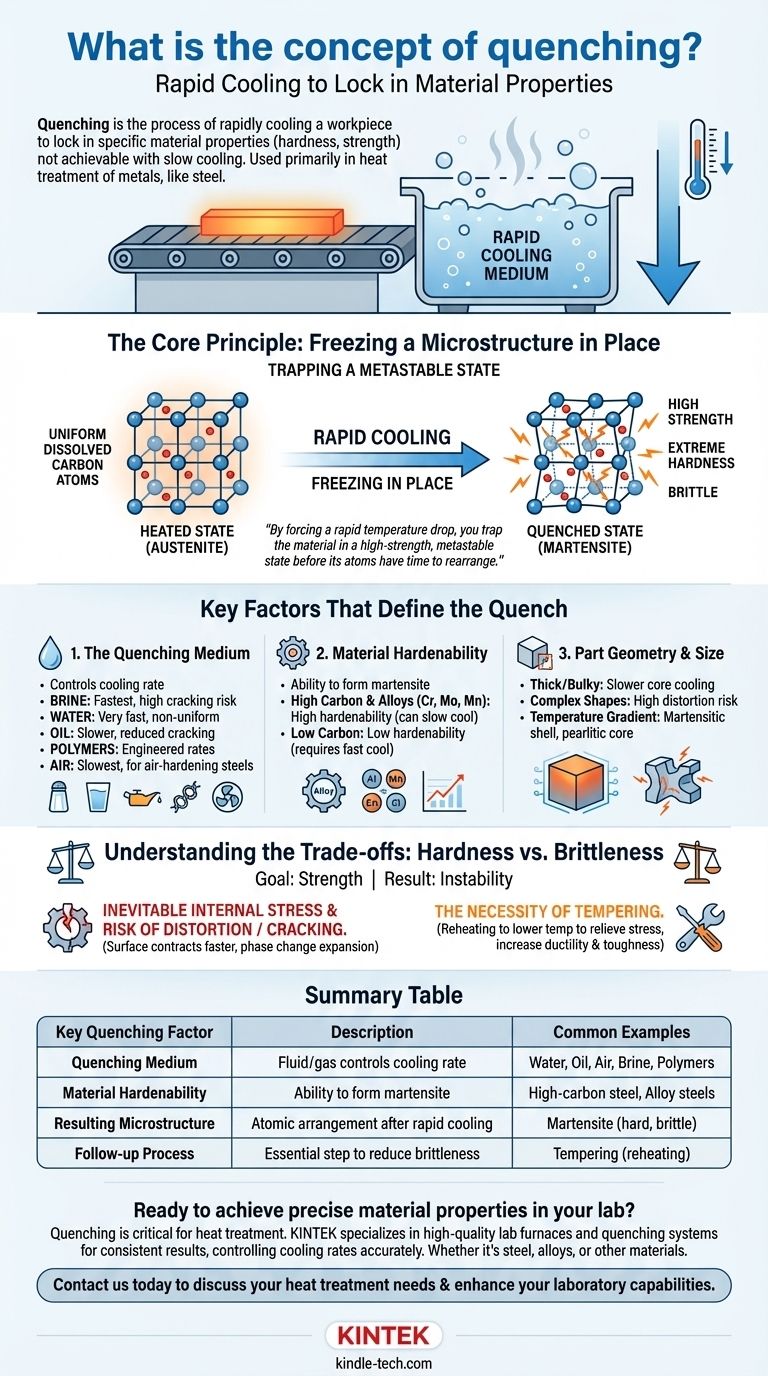
In materials science, quenching is the process of rapidly cooling a workpiece to lock in specific material properties that would not be achievable with slow cooling. It is a cornerstone of heat treatment, used primarily to increase the hardness and strength of metals, most notably steel. The process involves heating the material to a specific temperature and then plunging it into a medium like water, oil, or air.
Quenching is not merely about cooling; it is a controlled manipulation of a material's atomic structure. By forcing a rapid temperature drop, you trap the material in a high-strength, metastable state before its atoms have time to rearrange into a softer, more stable form.

The Core Principle: Freezing a Microstructure in Place
The purpose of quenching is to intentionally create and preserve a specific atomic arrangement, or microstructure, that yields desirable mechanical properties.
Heating to Create a Uniform State
Before quenching, a metal like steel is first heated to a critical temperature in a process called austenitizing. At this high temperature, the steel's crystalline structure changes into a phase called austenite, which has the unique ability to dissolve carbon atoms into a uniform solid solution. This creates a homogenous, high-energy starting point.
The Critical Role of Rapid Cooling
Once the material is uniformly in its austenite phase, the rapid cool of the quench begins. The cooling rate is so fast that the carbon atoms dissolved in the structure do not have time to diffuse out and form softer structures like pearlite or bainite.
The atoms are essentially "frozen" in place. This traps the crystal structure in a highly strained, supersaturated state, forcing it into a new microstructure that only exists because of this rapid transformation.
The Result: Creating Martensite in Steel
In steel, this new, trapped microstructure is called martensite. Martensite is extremely hard and strong because its distorted, body-centered tetragonal (BCT) crystal structure resists dislocation movement, which is the primary mechanism of plastic deformation in metals.
However, this extreme hardness comes at a cost: as-quenched martensite is also very brittle and contains significant internal stresses.
Key Factors That Define the Quench
The outcome of quenching is not a single result but a spectrum of possibilities controlled by several critical variables.
The Quenching Medium
The type of fluid or gas used for cooling—the quenchant—is the most significant factor controlling the cooling rate.
- Brine (salt water): Provides the fastest cooling rate due to suppressed vapor bubble formation but carries a very high risk of distortion and cracking.
- Water: Offers a very fast cool but can lead to non-uniform cooling and high internal stress.
- Oil: Cools significantly slower than water, reducing the risk of cracking. It is one of the most common quenchants for alloy steels.
- Polymers: Polymer solutions in water can be engineered to provide cooling rates between that of water and oil.
- Air: Provides the slowest quench. This is only effective for highly alloyed "air-hardening" steels that are designed to form martensite even with slow cooling.
The Material's Hardenability
Hardenability is a measure of a material's ability to form martensite upon cooling. A steel with high carbon and alloy content (like chromium, molybdenum, or manganese) has high hardenability. This means it can be cooled more slowly (e.g., in oil or even air) and still achieve full hardness deep into its core. Low-carbon steels have low hardenability and require a very fast quench (water) to become hard, and even then, only on the surface.
Part Geometry and Size
A thick, bulky component will always cool more slowly at its core than at its surface. This temperature gradient can result in a hard, martensitic shell with a softer, pearlitic core. This is a critical consideration in designing structural parts, as the properties will not be uniform throughout the cross-section.
Understanding the Trade-offs: Hardness vs. Brittleness
Quenching is a powerful process, but it introduces a fundamental trade-off that must be managed. The goal is strength, but the immediate result is often instability.
The Inevitable Rise in Internal Stress
When a part is quenched, the surface cools and contracts much faster than the interior. This differential cooling and the phase transformation to martensite (which involves a slight volume expansion) generate immense internal stresses within the material.
The Risk of Distortion and Cracking
If these internal stresses exceed the material's strength, the part will either distort (warp) or, in the worst case, crack. This is the primary risk associated with quenching, especially when using aggressive quenchants on complex shapes or high-carbon steels.
The Necessity of Tempering
Because of its extreme brittleness and high internal stress, a quenched part is rarely used in its "as-quenched" state. It is almost always followed by a second heat treatment process called tempering.
Tempering involves reheating the quenched part to a much lower temperature (e.g., 200-650°C or 400-1200°F). This process relieves internal stresses and allows some carbon to precipitate out, increasing the material's ductility and toughness while only moderately decreasing its hardness.
Making the Right Choice for Your Goal
The ideal quenching strategy is dictated entirely by the final properties your component requires.
- If your primary focus is maximum surface hardness: Use a fast quench (water/brine) on a suitable steel, but know that a subsequent tempering step is mandatory to reduce brittleness for nearly all applications.
- If your primary focus is balancing strength and toughness: Choose an alloy steel with higher hardenability and use a less severe quench (oil) to manage internal stress and reduce the risk of cracking.
- If your primary focus is minimizing distortion on a complex part: Select an air-hardening tool steel that is specifically designed to achieve high hardness with the slow cooling rate of an air quench.
Ultimately, mastering quenching is about controlling the cooling rate to achieve a precise and predictable balance between strength, toughness, and dimensional stability.
Summary Table:
| Key Quenching Factor | Description | Common Examples |
|---|---|---|
| Quenching Medium | Fluid or gas that controls cooling rate | Water, Oil, Air, Brine, Polymers |
| Material Hardenability | Ability to form martensite upon cooling | High-carbon steel, Alloy steels |
| Resulting Microstructure | Atomic arrangement after rapid cooling | Martensite (hard, brittle) |
| Follow-up Process | Essential step to reduce brittleness | Tempering (reheating to lower temperature) |
Ready to achieve precise material properties in your lab?
Quenching is a critical step in heat treatment, and having the right equipment is essential for consistent, reliable results. At KINTEK, we specialize in high-quality lab furnaces and quenching systems designed for materials science and metallurgy. Whether you're working with steel, alloys, or other materials, our solutions help you control cooling rates accurately to achieve the perfect balance of hardness and toughness.
Contact us today to discuss your specific heat treatment needs and discover how KINTEK can enhance your laboratory's capabilities. Get in touch via our contact form – let's build stronger materials together.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- Can I vacuum the inside of my furnace? A Guide to Safe DIY Cleaning vs. Professional Service
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost
- Why do you vacuum for heat treatment? Achieve Flawless, High-Performance Metal Components
- What is vacuum heat treatment process? Achieve Superior Control, Cleanliness, and Quality
- What are the uses of vacuum furnace? Achieve Unmatched Material Purity and Performance



















