In practice, graphite is considered a material with high electrical and thermal conductivity. While its exact conductivity varies significantly with its form, purity, and orientation, its ability to conduct electricity is a defining characteristic, stemming from a unique atomic structure that gives it properties similar to metals in some respects and ceramics in others.
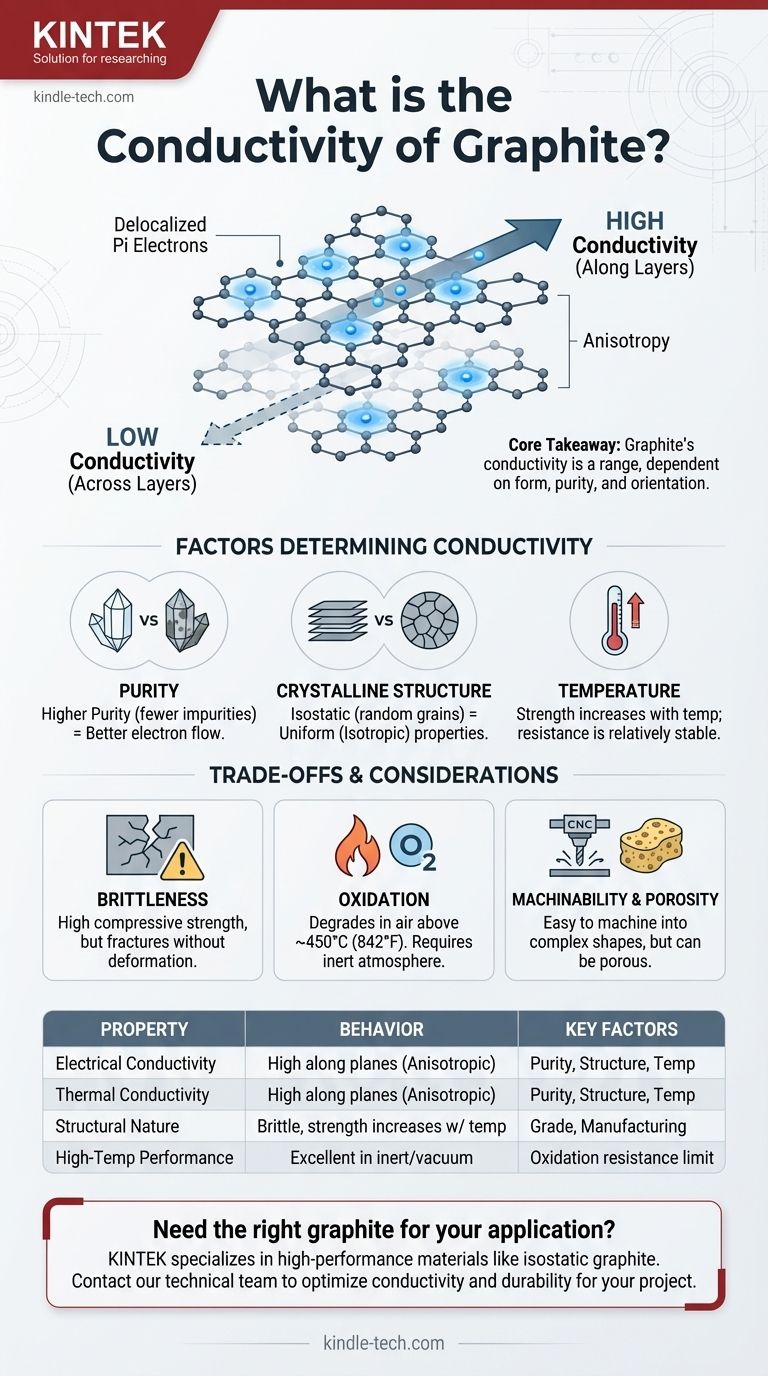
The core takeaway is that graphite's conductivity is not a single value but a range of behaviors. Its unique layered carbon structure allows electrons to move freely along its planes, creating high conductivity, but this property is highly dependent on the material's specific grade, purity, and crystalline orientation.

The Source of Graphite's Conductivity
To understand why graphite conducts electricity, we must look at its atomic-level structure. It is fundamentally different from most other non-metals.
A Unique Atomic Structure
Graphite consists of carbon atoms arranged in a hexagonal lattice. These lattices form vast, two-dimensional sheets, often compared to layers of chicken wire, that are stacked on top of each other.
Delocalized Pi Electrons
Within each of these layers, every carbon atom is bonded to three others. This leaves one outer-shell electron—a pi electron—unbonded. These electrons are "delocalized," meaning they are not tied to a single atom and can move freely along the entire layer. This sea of mobile electrons is precisely what allows graphite to conduct electricity so effectively, much like electrons in a metal.
Anisotropy: Why Direction Matters
The weak forces holding these layers together, however, do not allow electrons to jump between them easily. This creates a property called anisotropy, where a material's properties differ based on direction.
Graphite's electrical and thermal conductivity are extremely high along the layers but very low across them. This is a critical factor in any advanced application.
Factors That Determine Final Conductivity
Not all graphite is the same. Commercially available forms, such as the isostatic graphite mentioned in technical specifications, are engineered for specific performance characteristics.
The Role of Purity
As with any conductor, impurities disrupt the flow of electrons. The highest-purity graphite, with impurity levels below 5 parts per million (ppm), offers the best potential for high conductivity because the path for electrons is less obstructed.
The Impact of Crystalline Structure
In a perfect single crystal of graphite, anisotropy is extreme. However, most industrial forms, like isostatic graphite, are polycrystalline.
Isostatic graphite is formed under high pressure from all directions, creating a material with millions of tiny graphite crystals (grains) that are randomly oriented. This process averages out the directional properties, resulting in a material with more uniform, or isotropic, electrical and thermal conductivity in all directions.
The Influence of Temperature
While many materials lose conductivity as they heat up, graphite exhibits unusual behavior. Its mechanical strength actually increases with temperature up to a certain point. Its electrical resistance is also relatively stable compared to metals, making it suitable for high-temperature electrical applications like furnace elements.
Understanding the Trade-offs
Graphite's unique combination of properties comes with important limitations that must be considered in any design.
Mechanical Brittleness vs. Strength
Although its compressive strength is high and increases with temperature, graphite is a brittle material. Unlike metals, it will fracture without deforming under high impact or tensile stress.
Oxidation in Atmosphere
Graphite has excellent resistance to thermal shock and performs well at extreme temperatures, but this is typically in a vacuum or an inert atmosphere. When exposed to oxygen at high temperatures (generally above 450°C or 842°F), it will begin to oxidize and degrade.
Machinability and Porosity
One of graphite's greatest advantages is its ease of machining into complex shapes. However, depending on the grade and manufacturing process, it can have a certain level of porosity, which can be a concern in high-vacuum or ultra-pure applications where outgassing or contamination is a risk.
Making the Right Choice for Your Goal
The "best" graphite is the one that is optimized for your specific engineering challenge.
- If your primary focus is maximum electrical conductivity: Seek out high-purity, highly crystalline grades of graphite, and be prepared to manage the challenges of its anisotropic (directional) behavior.
- If your primary focus is uniform, predictable performance: Isostatic graphite is the superior choice, as its random grain orientation provides consistent thermal and electrical properties in all directions.
- If your primary focus is high-temperature electrical applications: Graphite's low electrical resistance, high thermal shock resistance, and increasing strength with temperature make it an ideal candidate, provided the atmosphere is controlled to prevent oxidation.
Ultimately, leveraging graphite's power comes from understanding that its form dictates its function.
Summary Table:
| Property | Behavior in Graphite | Key Influencing Factors |
|---|---|---|
| Electrical Conductivity | High along crystal planes (anisotropic) | Purity, crystalline structure (e.g., isotropic vs. anisotropic), temperature |
| Thermal Conductivity | High along crystal planes (anisotropic) | Purity, crystalline structure, temperature |
| Structural Nature | Brittle, but strength increases with temperature | Grade, manufacturing process (e.g., isostatic pressing) |
| High-Temperature Performance | Excellent in inert/vacuum atmospheres | Oxidation resistance above ~450°C (842°F) |
Need the right graphite for your specific application?
Graphite's performance is highly dependent on its grade, purity, and structure. The experts at KINTEK specialize in high-performance materials like isostatic graphite for laboratory and industrial equipment. We can help you select the ideal material to ensure optimal conductivity, thermal management, and durability for your project.
Contact our technical team today to discuss your requirements and leverage our expertise in lab materials and consumables.
Visual Guide
Related Products
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Is biomass conversion environmentally friendly? It Depends on Your Feedstock and Technology
- Is biomass renewable or renewable? A Deep Dive into Sustainable Energy's Carbon Cycle
- What are the three sample preparation techniques? Master the Key Stages for Accurate Analysis
- What is the difference between evaporation and sputtering in coating technology? Choose the Right Method for Your Lab
- What is the application of reactive sputtering? Synthesize High-Performance Compound Films
- What is the HIP process in casting? Achieve Dense, High-Performance Metal Components
- What is the product of physical vapor deposition? A High-Performance Thin Film Coating
- How does the working of oil-free diaphragm vacuum pumps differ from conventional pumps? A Guide to Clean vs. Deep Vacuum



















