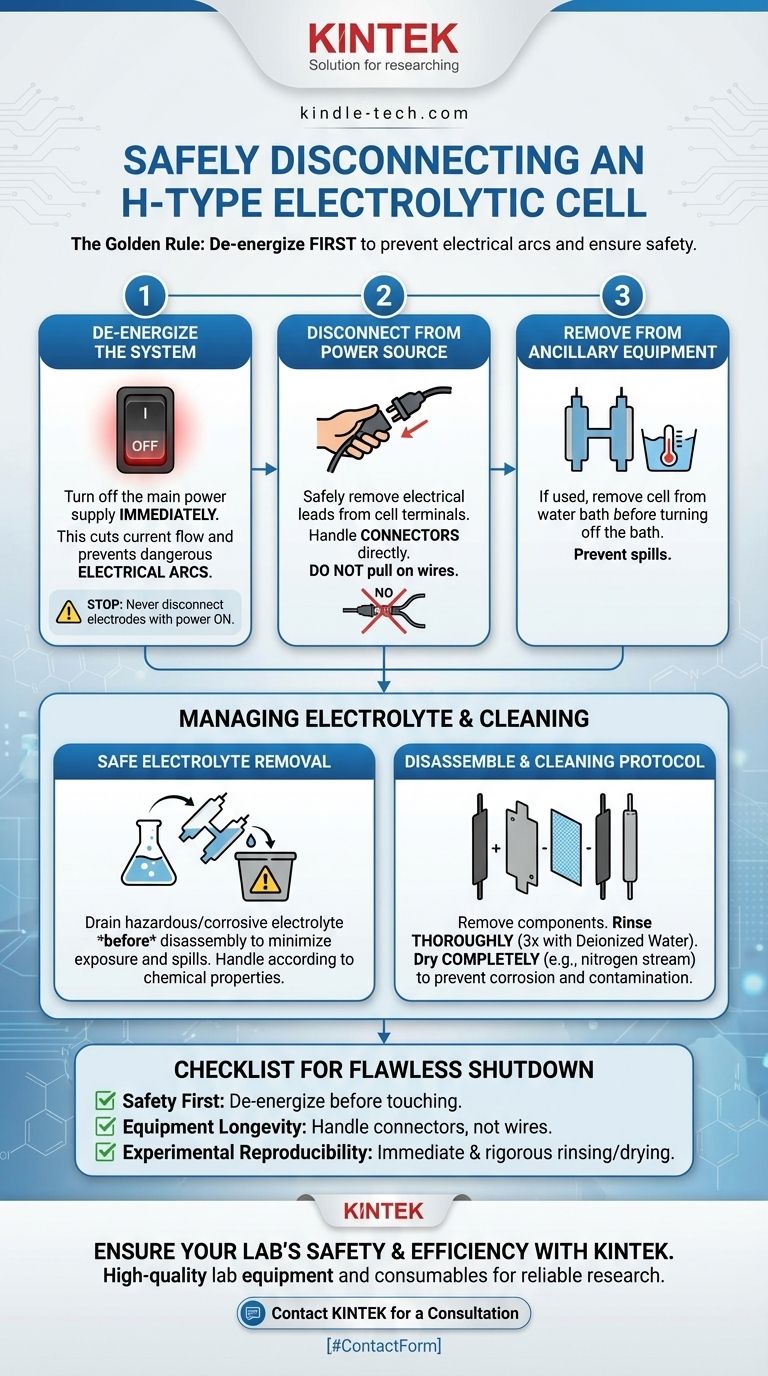
The correct procedure for disconnecting an H-type electrolytic cell always begins with one critical action: turning off the power supply before physically disconnecting any wires. This single step is the most important part of the process, designed to prevent electrical arcs and ensure your safety. Only after the system is fully de-energized should you proceed with physically disassembling the experimental setup.
The proper shutdown of an electrolytic cell is not just about turning it off. It is a systematic protocol that prioritizes personal safety from electrical and chemical hazards, preserves the integrity of your equipment, and ensures the validity of future experiments by preventing contamination.

The Core Shutdown Sequence: A Step-by-Step Guide
Following a specific order of operations is crucial for a safe and effective shutdown. Deviating from this sequence introduces unnecessary risk to both the operator and the equipment.
Step 1: De-energize the System
The absolute first step is to turn off the main power supply. This immediately cuts the flow of current to the cell.
Attempting to disconnect the electrodes while the power is still on can create a dangerous electrical arc as the connection is broken, posing a significant safety hazard.
Step 2: Disconnect from the Power Source
Once the power supply is confirmed to be off, you can safely disconnect the electrical leads from the terminals of the electrolytic cell.
When doing so, handle the connectors directly. Never pull on the wires, as this can damage the connection points on both the leads and the electrodes.
Step 3: Remove from Ancillary Equipment
If your cell is in a constant temperature water bath, remove the cell from the bath after disconnecting it from the power supply.
Only after the electrolytic cell has been safely removed should you turn off the water bath itself.
Handling the Cell and Electrolyte Safely
With the cell de-energized and disconnected, your focus shifts to managing the chemical components of the experiment safely.
The Importance of Safe Electrolyte Removal
If the electrolyte is corrosive, toxic, or hazardous, it should be handled with extreme care. It is often best to drain the electrolyte from the cell before removing the electrodes or disassembling the cell from its stand.
This practice minimizes the risk of spills and chemical exposure during disassembly. The removed electrolyte must be handled according to its chemical properties, which may involve neutralization, recycling, or disposal via approved waste streams.
Disassembling the Components
After the electrolyte is safely drained, you can proceed with removing the electrodes and other components, such as membranes or bridges.
Clean any residual liquid from the cell and its parts immediately to prevent corrosion or the drying of residues, which can be difficult to remove later.
Understanding the Risks and Common Pitfalls
Failing to follow the correct procedure can lead to preventable accidents and damaged equipment. Understanding these risks reinforces the importance of a disciplined approach.
The Risk of Electrical Arcs
The primary reason to turn off the power first is to prevent electrical arcing. An arc is a high-temperature plasma discharge that can cause severe burns and damage equipment terminals.
The Danger of Equipment Damage
Pulling on electrode wires can break internal connections, rendering expensive electrodes useless. Likewise, exposing the cell or its stand to corrosive electrolytes by mishandling them during disassembly will degrade your equipment over time.
The Hazard of Contamination
Improper cleaning is a common pitfall that compromises future experiments. Dried electrolyte residues or water stains can introduce impurities, leading to inaccurate and non-reproducible results.
Proper Cleaning for Future Experiments
A rigorous cleaning protocol is essential for maintaining the integrity of your H-cell and ensuring the quality of your research.
Immediate Rinsing Protocol
For experiments in aqueous solutions, the cell should be emptied and then immediately rinsed thoroughly at least three times with deionized water.
This prevents any dissolved salts or reagents from drying and crystallizing on the glass surfaces, which can be very difficult to remove once solidified.
Drying and Storage
After rinsing, the cell should be dried completely. A gentle stream of dry nitrogen or argon is an effective method for removing residual water without leaving stains or residue.
Once clean and dry, store the cell and its components in a safe, dust-free environment to prepare for the next experiment.
A Checklist for a Flawless Shutdown
Use this checklist to ensure you are covering all critical steps for safety, equipment preservation, and scientific accuracy.
- If your primary focus is personal safety: Always de-energize the power supply completely before your hands ever touch an electrical connection.
- If your primary focus is equipment longevity: Disconnect components by their connectors, not their wires, and immediately clean any corrosive residues.
- If your primary focus is experimental reproducibility: Implement a strict, immediate post-experiment cleaning protocol using deionized water and a proper drying method.
Following this disciplined procedure will protect you and your equipment while ensuring the integrity of your work.
Summary Table:
| Step | Key Action | Primary Purpose |
|---|---|---|
| 1. De-energize | Turn off main power supply | Prevent electrical arcing and ensure operator safety |
| 2. Disconnect | Remove electrical leads from terminals | Safely isolate the cell from the power source |
| 3. Remove Ancillary | Take cell out of water bath, then turn bath off | Prevent spills and manage auxiliary equipment safely |
| 4. Handle Electrolyte | Drain hazardous electrolyte before disassembly | Minimize chemical exposure and risk of spills |
| 5. Clean & Dry | Rinse with deionized water and dry completely | Prevent corrosion and contamination for future use |
Ensure Your Lab's Safety and Efficiency with KINTEK
Properly maintaining specialized equipment like H-type electrolytic cells is fundamental to laboratory safety and data integrity. At KINTEK, we understand the critical needs of researchers and lab technicians. We specialize in providing high-quality lab equipment and consumables designed for reliability and ease of use, helping you standardize procedures and protect your investments.
Whether you are setting up a new lab or optimizing your current processes, our expertise can support your work. Contact our specialists today to discuss your specific requirements and discover how our solutions can enhance your laboratory's performance and safety.
Contact KINTEK for a Consultation
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Double-Layer Water Bath Electrolytic Electrochemical Cell
People Also Ask
- What preparation steps are needed before starting an experiment with an H-type electrolytic cell? A Guide to Safe and Accurate Results
- What are the proper storage conditions for an H-type electrolytic cell? Ensure Long-Term Reliability and Accurate Results
- What experimental conditions need to be controlled when using an H-type electrolytic cell? Ensure Reliable and Repeatable Results
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What should be observed during an experiment with the H-type electrolytic cell? Key Monitoring for Precise Results



















