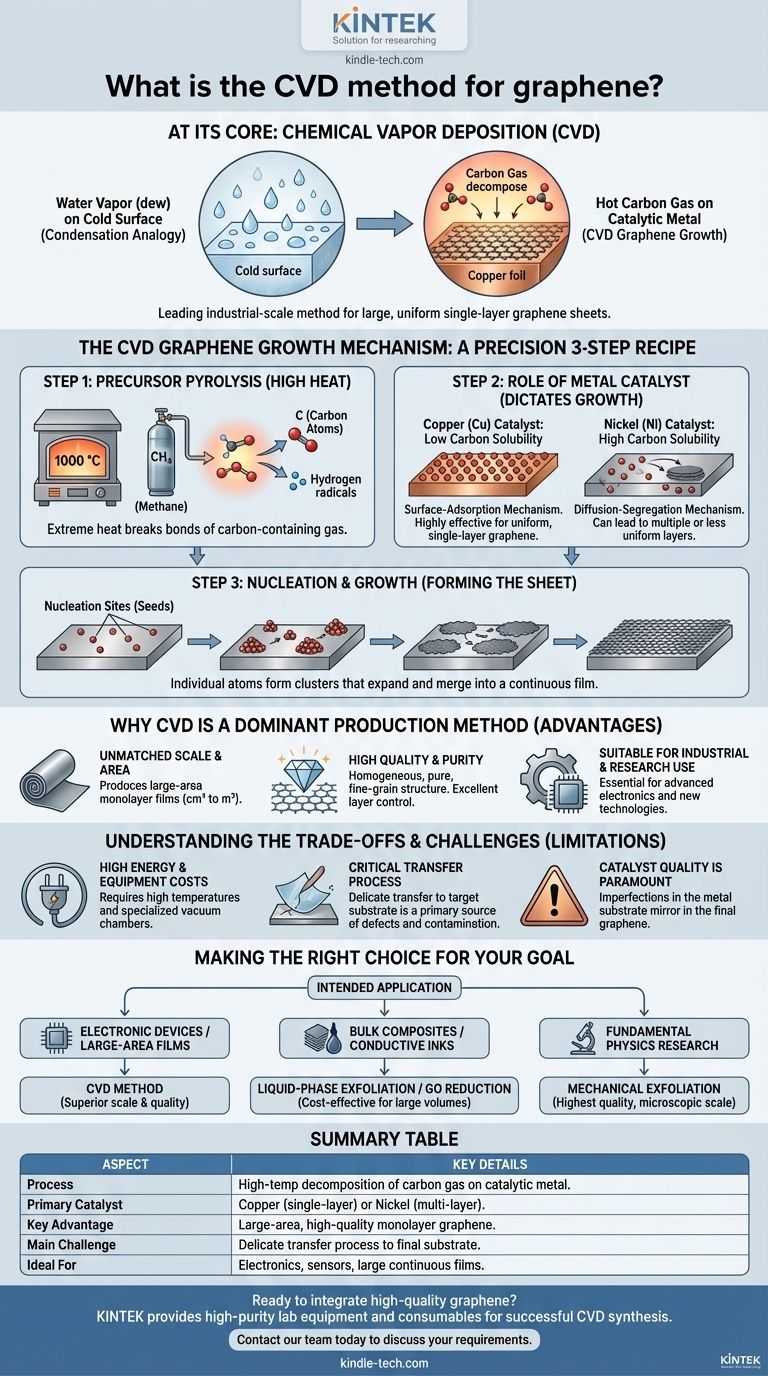
At its core, Chemical Vapor Deposition (CVD) for graphene is a synthesis method where a carbon-containing gas is heated in a chamber, causing it to decompose and "grow" a high-quality, single-atomic layer of graphene onto a metallic substrate. This process is analogous to condensation, but instead of water vapor forming dew on a cold surface, a hot carbon gas forms a solid film of graphene on a catalytic metal surface like copper.
Chemical Vapor Deposition is the leading industrial-scale method for producing large, uniform sheets of single-layer graphene. Its value lies not just in creating graphene, but in creating it with the scale and quality required for advanced electronic and materials applications, moving it from a lab curiosity to a viable technological component.

The CVD Graphene Growth Mechanism
To understand CVD, it is best to view it as a precise, three-step chemical recipe executed under controlled conditions. The quality of the final graphene sheet is entirely dependent on the control of each step.
Step 1: Precursor Pyrolysis
The process begins by feeding a hydrocarbon gas, such as methane (CH₄), into a high-temperature reactor, typically heated to around 1000 °C. This extreme heat provides the energy to break the chemical bonds of the gas molecules, a process known as pyrolysis. The gas decomposes into its constituent carbon atoms and other radicals.
Step 2: The Role of the Metal Catalyst
These free carbon atoms adsorb onto the surface of a metal substrate, which acts as a catalyst. The catalyst is the most critical component, as it dramatically lowers the energy required for the reaction and dictates the growth mechanism.
The choice of metal is key:
- Copper (Cu): Copper has very low carbon solubility. Carbon atoms adsorb directly onto the copper surface and arrange themselves into the graphene lattice. This is a surface-adsorption mechanism and is highly effective for growing uniform, single-layer graphene.
- Nickel (Ni): Nickel has high carbon solubility. Carbon atoms first dissolve into the bulk of the hot nickel. When the system is cooled, the carbon's solubility drops, and it precipitates back out onto the surface, forming graphene layers. This diffusion-segregation mechanism can sometimes lead to multiple or less uniform layers.
Step 3: Nucleation and Growth
On the catalyst surface, individual carbon atoms migrate and begin to form small, stable clusters. These clusters act as nucleation sites, or seeds, from which graphene crystals start to grow.
As more carbon atoms from the gas phase land on the surface, they attach to the edges of these growing islands. The islands expand and eventually merge, forming a continuous, seamless sheet of graphene across the entire surface of the catalyst substrate.
Why CVD is a Dominant Production Method
While other methods like mechanical exfoliation exist, CVD has become the standard for many applications due to several distinct advantages.
Unmatched Scale and Area
CVD is the most promising method for producing large-area monolayer graphene. Unlike exfoliation, which yields small, microscopic flakes, CVD can generate continuous graphene films measured in square centimeters or even meters, limited only by the size of the reactor and the substrate.
High Quality and Purity
When properly controlled, CVD yields exceptionally high-quality graphene. The resulting films exhibit high homogeneity, purity, and fine-grain structure. Critically, the process offers excellent control over the number of atomic layers, making it ideal for producing the single-layer sheets required for many electronic applications.
Suitability for Industrial and Research Use
The ability to produce large quantities of high-quality, large-area graphene makes the CVD method essential for both advanced research and the fabrication of next-generation technologies.
Understanding the Trade-offs and Challenges
Despite its advantages, the CVD method is not without its complexities and limitations. Objectivity requires acknowledging these practical hurdles.
High Energy and Equipment Costs
The process requires very high temperatures, making it energy-intensive. Furthermore, it relies on specialized equipment, including vacuum chambers and precision gas flow controllers, which represent a significant capital investment.
The Critical Transfer Process
Graphene grown via CVD forms on a metal catalyst, typically a thin foil. For most uses, it must be transferred from this metal foil to a target substrate, such as a silicon wafer. This delicate transfer step is a primary source of defects, wrinkles, tears, and contamination, which can degrade the graphene's pristine electronic properties.
Catalyst Quality is Paramount
The quality of the final graphene film is directly tied to the quality of the catalyst substrate. Imperfections, grain boundaries, or impurities on the copper or nickel foil will be mirrored in the resulting graphene sheet, impacting its uniformity and performance.
Making the Right Choice for Your Goal
Selecting a graphene production method depends entirely on the intended application and desired outcome.
- If your primary focus is electronic devices or large-area films: CVD is the superior method, providing the necessary scale and quality for fabricating transistors, sensors, and transparent conductive films.
- If your primary focus is creating bulk composites or conductive inks: Liquid-phase exfoliation or the reduction of graphene oxide are often more cost-effective for producing large volumes of graphene flakes needed for these applications.
- If your primary focus is fundamental physics research: Mechanical exfoliation of graphite can yield the highest-quality, defect-free graphene flakes, albeit on a microscopic scale, ideal for property characterization.
Ultimately, the CVD method is the critical bridge that allows graphene to move from laboratory potential to tangible, large-scale technology.
Summary Table:
| Aspect | Key Details |
|---|---|
| Process | High-temperature decomposition of carbon gas on a catalytic metal substrate. |
| Primary Catalyst | Copper (for single-layer) or Nickel (for multi-layer). |
| Key Advantage | Production of large-area, high-quality, uniform monolayer graphene. |
| Main Challenge | Delicate transfer process from the metal catalyst to the final substrate. |
| Ideal For | Electronics, sensors, and applications requiring large, continuous films. |
Ready to integrate high-quality graphene into your research or product development?
The CVD process is complex, but the payoff in material performance is immense. KINTEK specializes in providing the high-purity lab equipment and consumables—from reactor tubes to catalytic substrates—that are essential for successful and repeatable CVD graphene synthesis.
Let our experts help you build a reliable and efficient process. Contact our team today to discuss your specific requirements and how we can support your innovation.
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
People Also Ask
- Why are carbon nanotubes important in industry? Unlocking Next-Generation Material Performance
- How high of temperature do carbon nanotubes in air have the ability to sustain? Understanding the Oxidation Limit
- What is a CVD tube furnace? A Complete Guide to Thin-Film Deposition
- What are the advantages of industrial CVD for solid boriding? Superior Process Control and Material Integrity
- What function does CVD equipment serve in rhodium-modified coatings? Achieve Deep Diffusion and Microstructural Precision



















