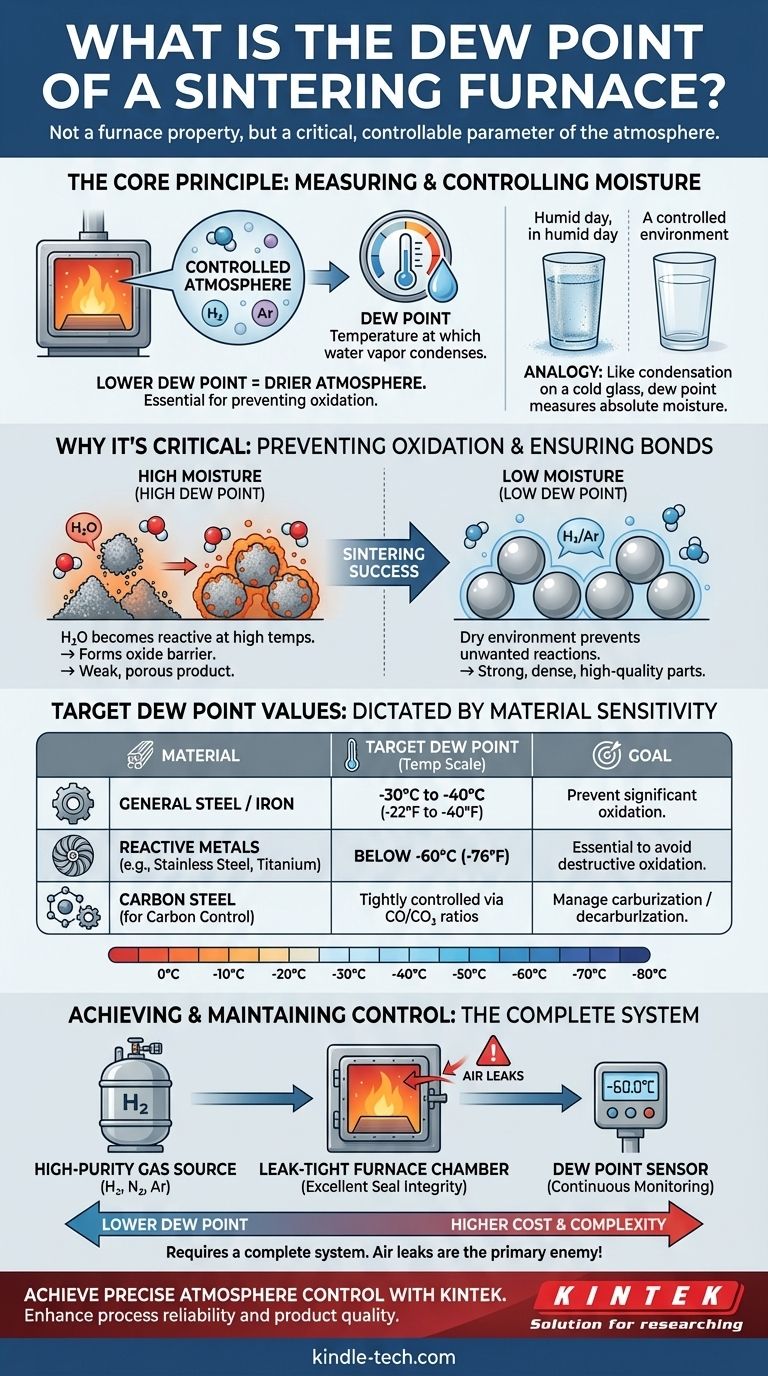
In the context of sintering, the dew point is not a fixed property of the furnace itself, but rather a critical, controllable parameter of the atmosphere inside it. It represents the temperature at which water vapor present in the furnace atmosphere would condense into liquid water. Therefore, the target dew point is set based on the specific material being processed, with lower dew points indicating a drier, more controlled atmosphere essential for preventing oxidation at high temperatures.
The core principle to understand is that dew point is the primary metric for measuring and controlling moisture in a furnace atmosphere. A low dew point is crucial because it signifies a dry environment that prevents unwanted chemical reactions, like oxidation, which can compromise the structural integrity of the sintered parts.

Why Dew Point is Critical in Sintering
Sintering is not just a thermal process; it is a thermochemical one. The atmosphere surrounding the parts is as important as the temperature, and moisture is one of the most damaging contaminants.
The Role of Atmosphere Control
The goal of a controlled atmosphere is to create a specific chemical environment. This environment should prevent oxidation of the metal powders and, in some cases, actively reduce any surface oxides that are already present.
Gases like dry hydrogen (H₂), dissociated ammonia (H₂-N₂), or inert gases like argon (Ar) are used. The purity of these gases is paramount.
How Moisture Causes Oxidation
At the extreme temperatures found in a sintering furnace, water vapor (H₂O) becomes highly reactive. It readily donates its oxygen atom to the metal parts you are trying to fuse together.
This forms metal oxides on the surface of the powder particles. These oxide layers act as a barrier, preventing the particles from properly bonding and densifying, resulting in a weak, porous final product.
Defining Dew Point in a Furnace Context
Think of a cold glass on a humid day—water condenses on the outside. The temperature of that glass is at or below the dew point of the surrounding air.
In a furnace, the dew point is a direct measure of the absolute moisture content in the process gas. A dew point of -40°C means the gas is so dry that you would have to cool it to -40°C before any water would condense. A lower dew point always means less water vapor.
Target Dew Point Values for Different Processes
The required dew point is dictated entirely by the material's sensitivity to oxygen. There is no single "correct" value; it depends on the metallurgical requirements.
For General Powder Metallurgy (e.g., Steel)
For common iron and steel-based components, a relatively dry atmosphere is sufficient. Preventing significant oxidation is the main goal.
A typical target dew point for sintering steel is in the range of -30°C to -40°C (-22°F to -40°F).
For Reactive and High-Affinity Metals
Materials like stainless steel, titanium, aluminum, and certain specialty alloys have a very high affinity for oxygen. Even trace amounts of moisture can cause destructive oxidation at sintering temperatures.
For these materials, an extremely dry atmosphere is non-negotiable. Target dew points are often below -60°C (-76°F), which requires high-purity gases and a furnace with exceptional integrity, often a vacuum furnace that is backfilled with purified gas.
For Controlling Carbon Potential
In more advanced applications, like sintering carbon steels, the dew point is monitored alongside CO/CO₂ ratios. This balance determines the "carbon potential" of the atmosphere—whether it will add carbon to (carburize) or remove carbon from (decarburize) the steel parts. Precise dew point control is essential for achieving the final material hardness.
Understanding the Trade-offs and Challenges
Achieving and maintaining a low dew point is a significant technical challenge that directly impacts furnace design, cost, and operation.
Achieving and Measuring Low Dew Points
A low dew point is not achieved by the furnace alone. It requires a complete system, including a source of high-purity process gas, leak-tight gas delivery lines, and a furnace chamber with excellent seal integrity.
Specialized dew point sensors (hygrometers) must be installed to continuously monitor the atmosphere and ensure the process stays within specification.
Cost vs. Purity
The drier the gas, the more expensive it is to purchase or generate on-site. Likewise, furnaces built to be "leak-tight" and capable of holding a high vacuum, as noted in their design features, are significantly more expensive. The cost of achieving a -60°C dew point is substantially higher than that for -30°C.
The Danger of Air Leaks
The primary enemy of a low dew point is an air leak. A tiny breach in a door seal, a fitting, or a weld can allow ambient, moisture-laden air to be drawn into the furnace. This can instantly raise the dew point, potentially ruining an entire batch of expensive parts. This is why furnace build quality and regular maintenance are so critical.
Making the Right Choice for Your Goal
Your target dew point is a direct function of your material and quality requirements.
- If your primary focus is sintering common steels or iron parts: A well-maintained furnace using a standard dry atmosphere with a dew point around -40°C is typically sufficient.
- If your primary focus is sintering oxygen-sensitive materials like stainless steel or titanium: You must invest in a high-integrity or vacuum furnace system capable of maintaining a dew point below -60°C.
- If your primary focus is ensuring process consistency and quality control: Continuous, real-time monitoring of the furnace dew point is more important than any single target value, as it allows you to detect problems like leaks immediately.
Ultimately, controlling the dew point is fundamental to controlling the chemistry of your sintering process and ensuring a successful outcome.
Summary Table:
| Material Type | Typical Target Dew Point | Key Consideration |
|---|---|---|
| General Steel / Iron | -30°C to -40°C (-22°F to -40°F) | Prevents significant oxidation |
| Reactive Metals (Stainless Steel, Titanium) | Below -60°C (-76°F) | Essential to avoid destructive oxidation |
| Carbon Steel (for Carbon Control) | Tightly controlled based on CO/CO₂ ratios | Manages carburization/decarburization |
Achieve Precise Atmosphere Control with KINTEK
Controlling the dew point in your sintering furnace is not just a technical detail—it's the difference between a high-quality, structurally sound product and a batch of failed parts. KINTEK specializes in lab equipment and consumables, providing robust solutions for maintaining the dry, controlled atmospheres essential for sintering success.
Our expertise ensures your laboratory can:
- Prevent Oxidation: Maintain ultra-low dew points to protect oxygen-sensitive materials.
- Ensure Process Consistency: Implement real-time monitoring for immediate leak detection and quality control.
- Optimize for Your Materials: From common steels to reactive alloys, get the right atmosphere for your specific needs.
Don't let moisture compromise your results. Contact KINTEK today to discuss how our sintering furnace solutions can enhance your process reliability and product quality.
Visual Guide
Related Products
- Vacuum Heat Treat and Pressure Sintering Furnace for High Temperature Applications
- Spark Plasma Sintering Furnace SPS Furnace
- Graphite Vacuum Furnace Negative Material Graphitization Furnace
- Horizontal High Temperature Graphite Vacuum Graphitization Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What temperature does titanium vaporize at? Unlocking Its Extreme Heat Resistance for Aerospace
- What is a sputtering machine? A Guide to High-Quality Thin Film Deposition
- What are the challenges of welding stainless steel? Overcome Warping, Sensitization, and Contamination
- How does a sputtering machine work? Achieve Atomic-Level Precision for Your Coatings
- What are the stages of sintering? A Guide to Mastering the Powder-to-Part Process



















