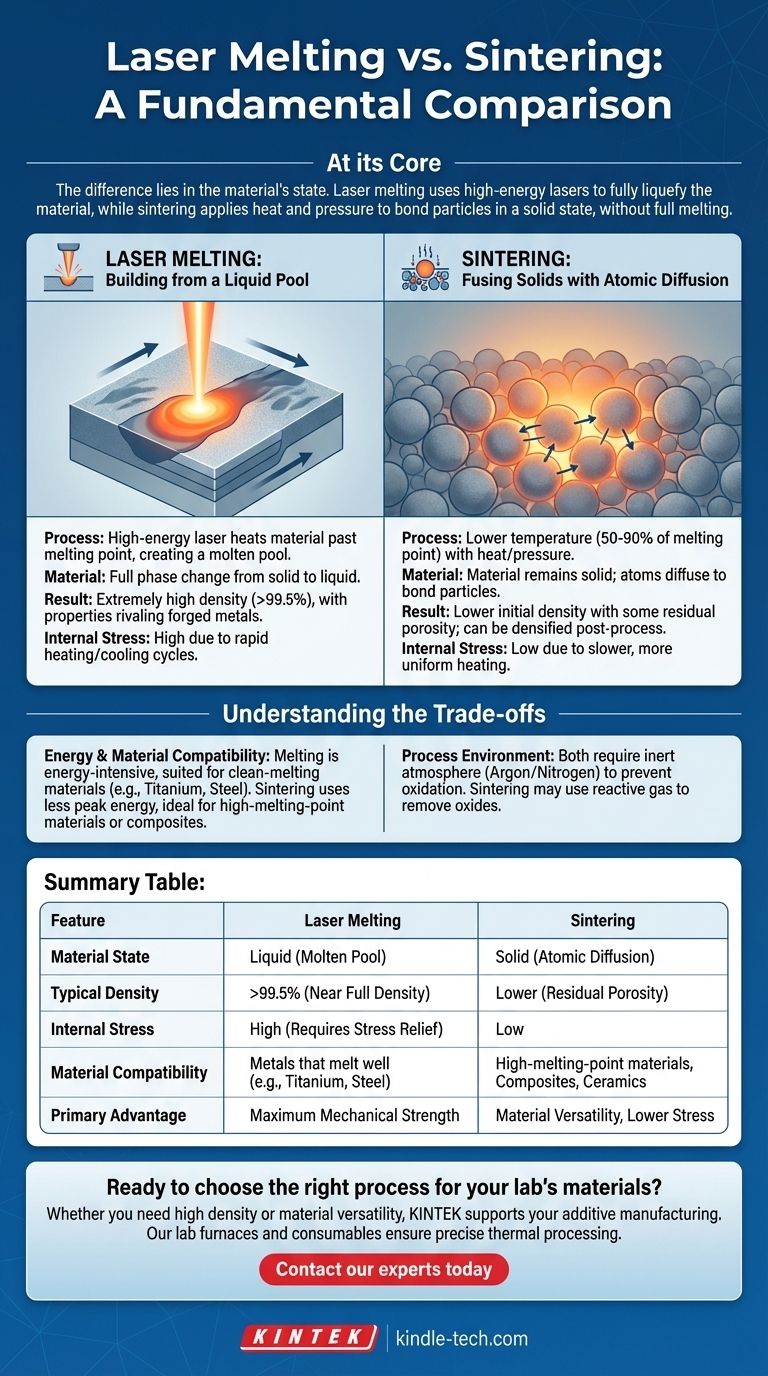
At its core, the difference between laser melting and sintering comes down to the state of the material. Laser melting uses a high-energy laser to heat a material past its melting point, creating a complete phase change from solid to liquid. Sintering, in contrast, uses heat and often pressure to bond particles together in a solid state, without ever fully liquefying the material.
While both processes bind powders into a solid object, the choice between them is a fundamental decision in materials engineering. Melting builds parts from a liquid state for maximum density, while sintering fuses particles in a solid state, opening a path for materials that are difficult or impossible to melt.

The Fundamental Difference: State of Matter
The distinction between melting and sintering is not just academic; it dictates the entire manufacturing process, the properties of the final part, and the types of materials you can use.
Laser Melting: Building from a Liquid Pool
In processes like Selective Laser Melting (SLM), a focused, high-power laser scans a bed of fine metal powder. The energy is so intense that it instantly melts the powder particles in its path, creating a tiny, localized molten pool.
This molten pool then cools and solidifies, fusing to the layer below it. The machine deposits a new layer of powder, and the process repeats, building a fully dense object layer-by-layer as if it were being micro-welded into existence.
Sintering: Fusing Solids with Atomic Diffusion
Sintering operates at a lower temperature, typically between 50% and 90% of the material's melting point. At this temperature, the material remains solid, but the atoms at the surface of each powder particle become highly agitated.
This thermal energy, often combined with external pressure, causes atoms to migrate across the boundaries between particles. This process, called solid-state diffusion, creates strong metallurgical bonds, gradually eliminating the voids between particles and consolidating the powder into a coherent mass.
How This Impacts the Final Part
The method used to fuse the powder has direct consequences for the final part's density, internal stress, and overall performance.
Density and Porosity
Laser melting generally produces parts with extremely high density, often exceeding 99.5%. Because the material is fully liquefied, it fills nearly all voids before solidifying, resulting in properties that can rival traditionally forged or cast metals.
Sintering, on the other hand, can leave some residual porosity in the final part. While techniques like Hot Isostatic Pressing (HIP) can be used after sintering to achieve full density, the initial sintered part is not inherently as dense as a melted one.
Material Properties and Internal Stress
The rapid heating and cooling cycles inherent in laser melting can introduce significant internal stresses within a part. These stresses often must be relieved through post-process heat treatments to prevent warping or cracking and achieve the desired mechanical properties.
Sintering is a slower, more uniform heating process. This gentler thermal cycle typically results in parts with much lower internal stress, simplifying post-processing requirements.
The "DMLS" Naming Confusion
It is critical to address a common point of confusion in the industry: Direct Metal Laser Sintering (DMLS). Despite its name, DMLS is a powder bed fusion process that involves localized melting or partial melting, not true solid-state sintering. The term is a brand name that has become synonymous with metal 3D printing, but the underlying physics are those of melting.
True sintering is a distinct thermal process, often used as a secondary step in other additive technologies like Binder Jetting, where a "green part" is first printed with a binding agent and then placed in a furnace to be sintered into a final, dense metal part.
Understanding the Trade-offs
Choosing a process requires weighing the benefits of part performance against the constraints of the material and application.
Energy and Material Compatibility
Melting is an energy-intensive process that requires a system capable of delivering and managing very high temperatures. It is best suited for materials that can be cleanly melted and solidified, like titanium alloys, stainless steels, and aluminum.
Sintering requires less peak energy and is the only viable path for materials with exceptionally high melting points (like tungsten or certain ceramics) or for composites where one material would be destroyed by the melting temperature of the other.
The Role of Process Environment
The environment in which the process occurs is critical. To prevent oxidation at high temperatures, both melting and sintering of metals require a tightly controlled, inert atmosphere (typically argon or nitrogen).
For certain materials, sintering may even require a reactive gas environment (like hydrogen) to remove surface oxides from the powder particles and promote effective atomic bonding.
Making the Right Choice for Your Goal
Your application's primary requirement—be it mechanical performance, material choice, or production cost—should guide your decision.
- If your primary focus is maximum density and mechanical strength: Choose a laser melting process (SLM/DMLS) to create near-fully dense parts with properties comparable to wrought metals.
- If your primary focus is working with high-temperature ceramics or specialized metal matrix composites: True sintering is often the superior or only viable method for consolidating materials that cannot be effectively melted.
- If your primary focus is cost-effective series production: Consider technologies like Binder Jetting, which rely on sintering as a secondary step, as they can offer higher throughput for the initial printing stage.
Understanding the physics of how particles are joined—either by liquefaction or atomic diffusion—is the key to selecting the right tool for your engineering challenge.
Summary Table:
| Feature | Laser Melting | Sintering |
|---|---|---|
| Material State | Liquid (Molten Pool) | Solid (Atomic Diffusion) |
| Typical Density | >99.5% (Near Full Density) | Lower (Residual Porosity) |
| Internal Stress | High (Requires Stress Relief) | Low |
| Material Compatibility | Metals that melt well (e.g., Titanium, Steel) | High-melting-point materials, Composites, Ceramics |
| Primary Advantage | Maximum Mechanical Strength | Material Versatility, Lower Stress |
Ready to choose the right process for your lab's materials?
Whether your project requires the high density of laser melting or the material versatility of sintering, KINTEK has the expertise and equipment to support your laboratory's additive manufacturing and materials research. Our range of lab furnaces and consumables is designed for precise thermal processing.
Contact our experts today to discuss how we can help you achieve your material property goals.
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Molybdenum Vacuum Heat Treat Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- What are some applications of brazing? Join Dissimilar Metals with Strong, Leak-Proof Bonds
- How is the greatest joint strength obtained in brazing? Master the 3 Keys to Superior Metallurgical Bonds
- Can dissimilar metals be brazed or braze welded? A Guide to Strong, Reliable Joints
- What are the advantages of brazing over braze welding? Achieve Stronger, Cleaner, and Repeatable Joints
- What is vacuum brazed? The Ultimate Guide to High-Purity Metal Joining



















