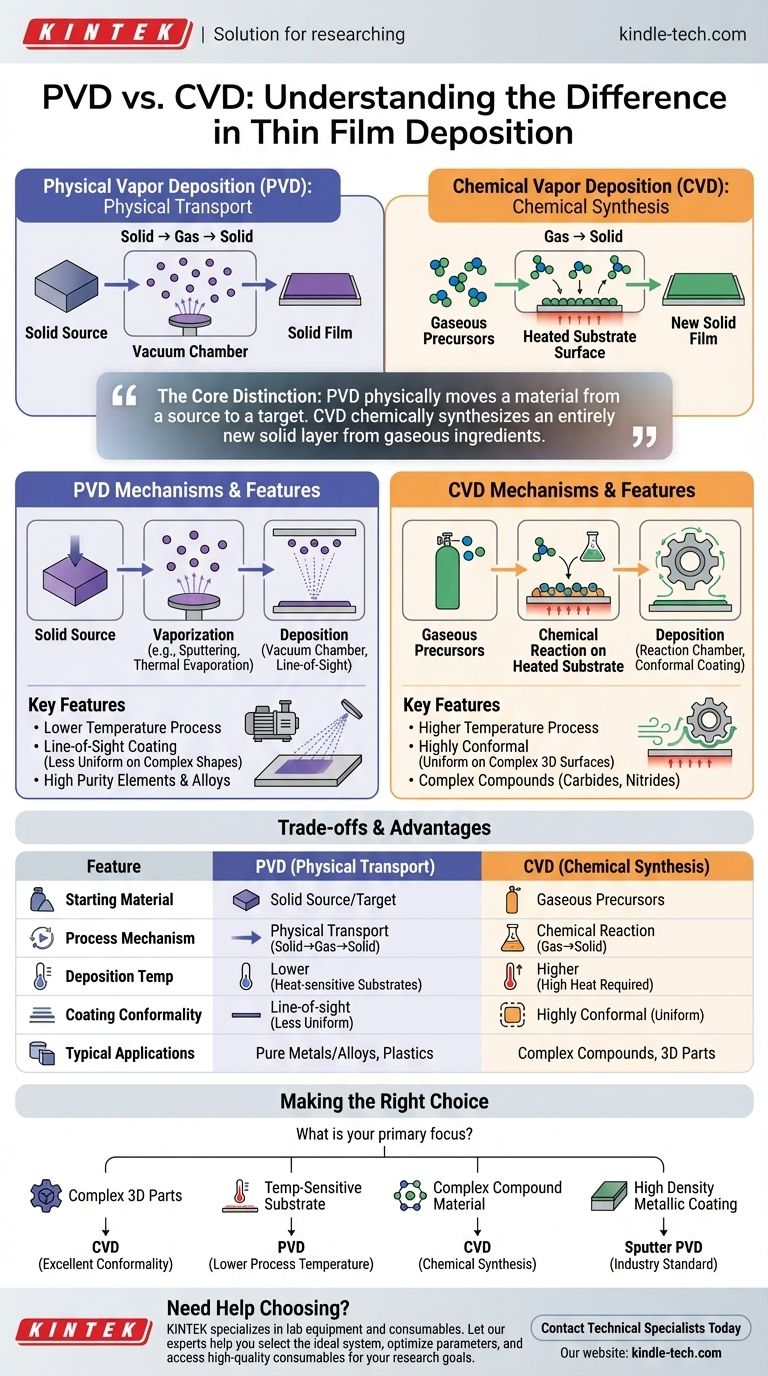
In essence, the primary difference lies in the state of the material before it is deposited onto a surface. Physical Vapor Deposition (PVD) involves vaporizing a solid material into a gas and then condensing it back into a solid thin film on a substrate. In contrast, Chemical Vapor Deposition (CVD) uses chemical reactions between gas precursors at the substrate's surface to create a new solid material directly on that surface.
The core distinction is simple: PVD physically moves a material from a source to a target. CVD chemically synthesizes an entirely new solid layer from gaseous ingredients.

The Mechanics of Physical Vapor Deposition (PVD)
Physical Vapor Deposition is fundamentally a transport process. It takes a material that already exists in a solid form, moves it atom by atom, and reassembles it elsewhere as a thin film.
The Core Principle: Solid to Gas to Solid
The material to be deposited, known as the source or target, is placed in a high-energy environment. This energy causes atoms or molecules to escape from the surface of the source, effectively turning it into a vapor.
This vapor then travels and condenses on a cooler surface, the substrate, forming the desired thin film.
Common PVD Methods
While the principle is the same, the method of vaporizing the source material can differ. The two most common methods are sputtering, where the source is bombarded with energetic ions, and thermal evaporation, where the source is heated until it vaporizes.
The Critical Role of the Vacuum
PVD processes are almost always conducted in a vacuum chamber. This is crucial because it removes air and other particles, allowing the vaporized atoms to travel freely from the source to the substrate without colliding with anything else.
The Chemistry of Chemical Vapor Deposition (CVD)
Chemical Vapor Deposition is a synthesis process. It does not start with the final material but rather creates it on the spot through controlled chemical reactions.
The Core Principle: Gas to Solid
In CVD, one or more volatile precursor gases are introduced into a reaction chamber. The process does not begin with a solid target of the desired material.
How the Film is Formed
The substrate is typically heated to a specific temperature. When the precursor gases come into contact with the hot substrate, they react or decompose, leaving behind a solid thin film. The byproducts of the reaction are then removed as gas.
Key Environmental Factors
CVD relies on precise control of variables like temperature, pressure, and gas flow rates. The final properties of the film are determined entirely by the chemistry occurring inside the chamber.
Understanding the Key Differences & Trade-offs
Choosing between PVD and CVD depends entirely on the material, the substrate, and the desired properties of the final film. Their different mechanisms lead to distinct advantages and disadvantages.
Starting Material: Solid vs. Gas
This is the most fundamental difference. PVD starts with a solid source, while CVD starts with gaseous precursors. This dictates the types of materials that can be deposited easily with each method.
Deposition Temperature: Lower vs. Higher
PVD is generally a lower-temperature process compared to most conventional CVD methods. This makes PVD suitable for coating materials that cannot withstand high heat, such as plastics.
Film Conformality: Line-of-Sight vs. Uniform
PVD is a "line-of-sight" technique. The vaporized atoms travel in straight lines, making it difficult to evenly coat complex, three-dimensional shapes.
CVD, however, is highly conformal. The precursor gases can flow around complex geometries, allowing for a uniform coating on all surfaces.
Purity and Complexity
PVD excels at depositing extremely pure films of elements or alloys, as it simply transports the source material. CVD can create more complex compounds, such as silicon nitride or tungsten carbide, that would be difficult to produce and then vaporize as a PVD source.
Making the Right Choice for Your Goal
Your application's specific requirements will dictate which method is superior.
- If your primary focus is uniform coverage on a complex 3D part: CVD is the better choice due to its excellent conformality.
- If your primary focus is depositing a pure metal or alloy onto a temperature-sensitive substrate: PVD is the superior option because of its lower processing temperatures.
- If your primary focus is creating a complex compound material like a carbide or nitride: CVD provides the chemical pathway to synthesize these materials directly on the substrate.
- If your primary focus is achieving the highest possible film density and adhesion for a metallic coating: Sputter PVD is often the industry standard.
Ultimately, understanding the mechanism—physical transport versus chemical creation—is the key to selecting the right tool for your engineering challenge.
Summary Table:
| Feature | Physical Vapor Deposition (PVD) | Chemical Vapor Deposition (CVD) |
|---|---|---|
| Starting Material | Solid source/target | Gaseous precursors |
| Process Mechanism | Physical transport (solid→gas→solid) | Chemical reaction (gas→solid) |
| Deposition Temperature | Lower (suitable for heat-sensitive substrates) | Higher |
| Coating Conformality | Line-of-sight (less uniform on complex shapes) | Highly conformal (uniform on all surfaces) |
| Typical Applications | Pure metals/alloys, temperature-sensitive substrates | Complex compounds (carbides, nitrides), 3D parts |
Need Help Choosing the Right Deposition Method for Your Lab?
Understanding the differences between PVD and CVD is crucial for achieving optimal results in your thin film applications. The right choice depends on your specific material, substrate, and performance requirements.
KINTEK specializes in lab equipment and consumables, serving all your laboratory needs. Our experts can help you:
- Select the ideal deposition system (PVD or CVD) for your specific application
- Optimize process parameters for superior film quality and adhesion
- Access a full range of high-quality consumables including targets and precursors
Let us help you make the right choice for your research or production goals. Contact our technical specialists today for personalized guidance and solutions tailored to your laboratory requirements.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- How does PECVD work? Enable Low-Temperature, High-Quality Thin Film Deposition
- What is the process of vacuum vapor deposition? Mastering CVD and PVD Thin-Film Coating
- What are the methods of deposition? A Guide to PVD and CVD Thin-Film Techniques
- What color diamonds are CVD? Understanding the Process from Brown Tint to Colorless Beauty
- What is the difference between PECVD and CVD? Unlock the Right Thin-Film Deposition Method



















