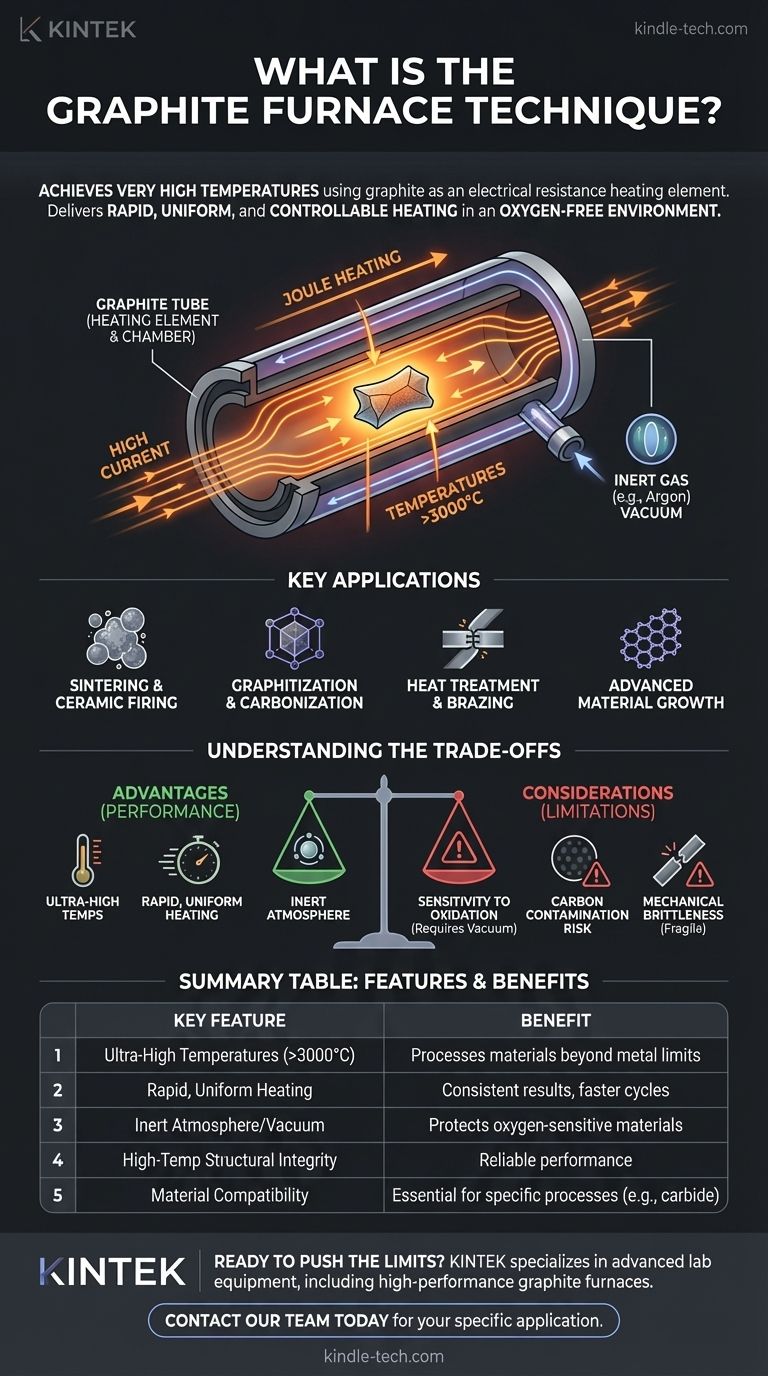
In essence, the graphite furnace technique is a method for achieving very high temperatures by using graphite as an electrical resistance heating element. Instead of using traditional metallic coils, an electric current is passed directly through a graphite structure—often a tube—which heats up rapidly and uniformly. This process is typically conducted within a vacuum or a controlled, protective atmosphere to prevent the graphite from oxidizing and to protect the material being processed.
The core value of the graphite furnace technique lies in its ability to deliver rapid, uniform, and controllable heating to temperatures well beyond the limits of most metallic elements. This makes it indispensable for manufacturing and research involving advanced materials, especially those requiring an oxygen-free environment.

How Graphite Furnaces Work: The Core Principle
To understand the applications, you must first grasp the fundamental mechanics. The technique’s advantages stem directly from the unique properties of graphite at extreme temperatures.
Graphite as a Resistance Heater
Graphite is an excellent conductor of electricity, but it still possesses electrical resistance. When a high current is passed through it, this resistance causes it to heat up intensely, a principle known as Joule heating. This allows for very fast heating and cooling rates compared to other furnace types.
Achieving High Temperature and Uniformity
Graphite maintains its structural integrity at temperatures exceeding 3000°C, far surpassing the melting point of conventional metal heating elements. Furnaces are often designed with a graphite tube that serves as both the heating element and the processing chamber, ensuring excellent temperature uniformity across the material inside.
The Critical Role of a Controlled Atmosphere
One of the most important operational aspects is the environment. At high temperatures, graphite will rapidly oxidize (burn) if exposed to air. Therefore, these furnaces must operate in a vacuum or be filled with an inert gas like argon. This not only protects the furnace components but also creates a pristine, oxygen-free environment for processing sensitive materials.
Key Applications Driven by Performance
The unique capabilities of graphite furnaces make them the preferred tool for a range of demanding, high-temperature industrial and research processes.
Sintering and Ceramic Firing
Sintering is the process of compacting and forming a solid mass of material by heat and pressure without melting it to the point of liquefaction. Graphite furnaces provide the high, uniform temperatures needed to sinter advanced ceramics and other powdered materials into dense, durable components.
Graphitization and Carbonization
These processes involve heating carbon-based materials to extreme temperatures to alter their crystalline structure. Graphitization converts amorphous carbon into crystalline graphite, while carbonization enriches a material's carbon content. These are foundational steps in producing high-performance carbon products.
Heat Treatment and Brazing
Processes like annealing (softening materials and relieving internal stresses), brazing (joining materials with a filler metal), and degassing (removing trapped gases from a material) benefit from the precise temperature control and clean, inert atmosphere of a graphite furnace.
Advanced Material Growth
Modern material science relies heavily on this technique. It is widely used for growing graphene, synthesizing carbon nanotubes, and producing specialized materials like silicon carbide, where purity and high temperatures are paramount.
Understanding the Trade-offs
While powerful, the graphite furnace technique is not a universal solution. Its operational requirements introduce specific limitations that you must consider.
Sensitivity to Oxidation
The absolute need for a vacuum or inert gas atmosphere is the most significant factor. This requirement adds complexity and cost to the system, as it necessitates vacuum pumps, gas management systems, and robust seals. Any leak can lead to the rapid degradation of the graphite elements.
Material Compatibility and Contamination
Carbon is reactive at high temperatures and can interact with the material being processed. This can be a desired effect, such as in carbide growth, but it can also be an unwanted source of carbon contamination in other applications. Careful selection of crucible materials is essential.
Mechanical Brittleness
Graphite is a brittle material. The heating elements and furnace insulation can be fragile and must be handled with care during installation, maintenance, and loading to prevent cracking or damage. This contrasts with the more ductile nature of many metallic heating elements.
Making the Right Choice for Your Process
Selecting the right heating technology depends entirely on your specific temperature, atmosphere, and material requirements.
- If your primary focus is reaching ultra-high temperatures (above 2000°C) for graphitization or advanced ceramic sintering: The graphite furnace is the industry-standard and often the only viable choice.
- If your primary focus is processing oxygen-sensitive materials or ensuring high purity: The inherent vacuum or inert atmosphere of a graphite furnace makes it a superior option.
- If your primary focus is lower-temperature processing (under 1200°C) in an open-air environment: A conventional furnace with metallic heating elements is a more practical and economical solution.
Ultimately, the graphite furnace is a specialized tool engineered for performance at the extremes of material processing.
Summary Table:
| Key Feature | Benefit |
|---|---|
| Ultra-High Temperatures (>3000°C) | Processes materials beyond the limits of metal elements |
| Rapid, Uniform Heating | Consistent results and faster processing cycles |
| Inert Atmosphere/Vacuum | Protects oxygen-sensitive materials and furnace components |
| High-Temperature Structural Integrity | Reliable performance for demanding applications |
| Material Compatibility Considerations | Essential for processes like carbide growth, but a risk for contamination |
Ready to push the limits of your high-temperature processing?
If your research or manufacturing involves sintering advanced ceramics, graphitization, heat treatment, or growing materials like graphene in an oxygen-free environment, the precise control of a graphite furnace is essential.
KINTEK specializes in advanced lab equipment, including high-performance graphite furnaces, to meet the demanding needs of modern laboratories and material science. Our experts can help you select the right system to achieve superior results.
Contact our team today to discuss your specific application and discover the KINTEK solution for you.
Visual Guide
Related Products
- Graphite Vacuum Continuous Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What does a graphite furnace do? Achieve Extreme Heat and Ultra-Sensitive Analysis
- What is the basic principle of graphite furnace atomic absorption spectroscopy? Achieve Ultra-Trace Element Detection
- What is the maximum working temperature of graphite? Unlock High-Temp Performance with the Right Atmosphere
- Why is a graphite furnace more sensitive than a flame? Unlocking Superior Trace Analysis
- Why is graphite furnace more sensitive than flame? Unlocking Ultra-Trace Detection for Your Lab
- What are the advantages of graphite furnace? Achieve High-Temperature Precision and Purity
- What are the advantages of graphite furnace AAS? Achieve Unmatched Sensitivity for Trace Element Analysis
- How does an induction graphitization furnace facilitate the transformation of unburned carbon into synthetic graphite?



















