The inert air technique, more accurately called an inert gas technique, is a set of procedures used to replace the reactive air inside a container with a non-reactive (inert) gas. This is done to handle and store chemical substances that would otherwise react with components of the air, such as oxygen or water vapor. The process typically involves repeated cycles of flushing the container with an inert gas, like nitrogen or argon, to dilute and displace the original atmosphere.
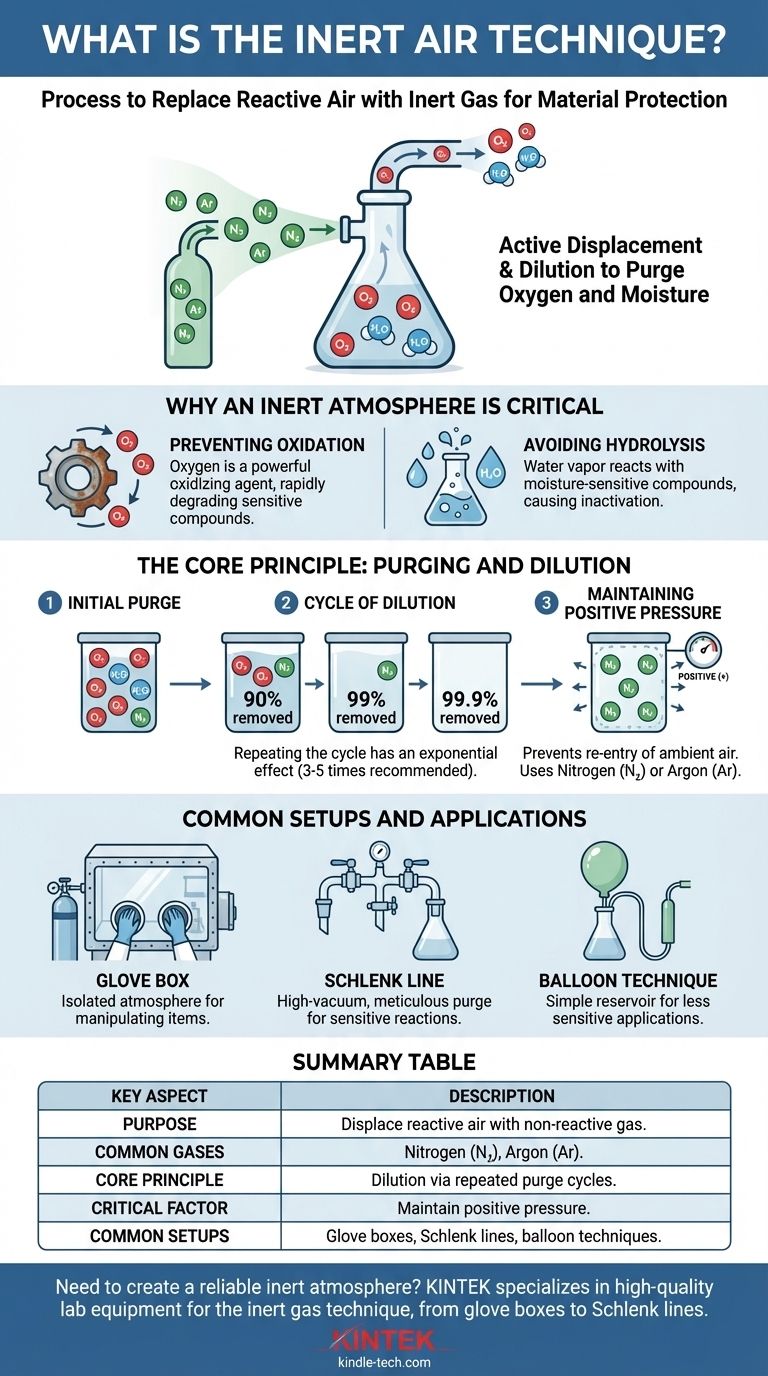
The core purpose of the inert air technique is not simply to add a non-reactive gas, but to systematically purge a workspace of oxygen and moisture. Understanding that this is a process of active displacement and dilution is the key to protecting sensitive materials from unwanted chemical reactions.

Why an Inert Atmosphere is Critical
Many chemical compounds are sensitive to their environment. The standard air we work in is approximately 21% oxygen and contains variable amounts of water vapor, both of which can be highly reactive.
Preventing Oxidation
Oxygen is a powerful oxidizing agent. Air-sensitive compounds can be rapidly degraded or transformed upon exposure, ruining experiments, changing chemical properties, and sometimes creating hazardous byproducts.
Avoiding Hydrolysis
Water vapor can react with moisture-sensitive (hygroscopic) compounds in a process called hydrolysis. This is especially problematic in organic and organometallic chemistry, where even trace amounts of water can inactivate reagents or catalyze unwanted side reactions.
The Core Principle: Purging and Dilution
The technique described in the reference—inflating and deflating a balloon—is a practical application of a fundamental principle: dilution. You are not creating a perfect vacuum; you are methodically reducing the concentration of reactive gases to a negligible level.
The Cycle of Dilution
Each time you flush a container with inert gas and vent it, you remove a large fraction of the original air. Repeating the cycle has an exponential effect.
For example, a single flush might remove 90% of the oxygen. The second flush removes 90% of the remaining 10%, and the third flush removes 90% of what's left after that. This is why repeating the cycle three to five times is a common rule of thumb to achieve a sufficiently inert atmosphere.
Maintaining Positive Pressure
Once the air is displaced, the goal is to prevent it from re-entering. This is achieved by maintaining a slight positive pressure of the inert gas inside the container. This ensures that if there are any small leaks, the inert gas will flow out rather than ambient air flowing in. A simple balloon attached to a flask serves this exact purpose.
Choosing the Right Gas
Nitrogen (N₂) and Argon (Ar) are the most common inert gases used. Nitrogen is less expensive and suitable for most applications. Argon is denser than air and more inert, making it the preferred choice for reactions involving metals that can react with nitrogen at high temperatures (like lithium). Using a "dry" grade of gas is crucial for moisture-sensitive work.
Common Setups and Applications
While the principle is universal, the application varies depending on the sensitivity of the materials and the scale of the work.
The Glove Box
A glove box is a sealed container with transparent panels and built-in gloves that allows you to manipulate items in an isolated atmosphere. The technique of purging it with nitrogen, as described in the reference, is common for creating the initial inert environment.
The Schlenk Line
A Schlenk line is a glass manifold used by chemists. It has a dual-vacuum and inert gas system, allowing a chemist to repeatedly evacuate the air from a flask with a vacuum pump and then backfill it with an inert gas, achieving a very high-quality inert atmosphere.
The Balloon Technique
For less sensitive applications, simply flushing a flask with inert gas from a tube and then capping it with a gas-filled balloon is sufficient. The balloon acts as a reservoir to maintain positive pressure as the flask cools or samples are taken.
Understanding the Trade-offs and Pitfalls
While powerful, these techniques are not foolproof. Understanding their limitations is key to successful execution.
It's About Reduction, Not Elimination
Standard purging techniques drastically reduce oxygen and moisture levels, often to parts-per-million levels. However, they do not create a perfect, 100.00% inert atmosphere. The final purity depends on the quality of your seals, the purity of your gas, and the number of purge cycles.
Leaks are the Enemy
The entire inert atmosphere can be instantly compromised by a single poorly sealed joint or a crack in the glassware. Maintaining a visible positive pressure (e.g., a slightly inflated balloon) is your best indicator that the system is properly sealed.
Not All Materials Are Equal
The required quality of the inert atmosphere depends entirely on what you are working with. A moderately air-sensitive organic compound may be fine with a simple balloon, whereas a pyrophoric compound (which ignites spontaneously in air) requires a much more rigorous setup like a high-integrity glove box.
Making the Right Choice for Your Goal
To apply this effectively, match the rigor of your technique to the sensitivity of your materials.
- If your primary focus is basic protection for moderately sensitive materials: A few purge cycles in a flask or simple glove box, followed by maintaining a positive pressure with a balloon, is often sufficient.
- If your primary focus is handling highly reactive or pyrophoric compounds: A more robust system like a Schlenk line or a high-integrity, continuously purged glove box is non-negotiable.
- If your primary focus is ensuring a moisture-free (anhydrous) environment: Using a high-purity, dry grade of inert gas and meticulously drying all glassware before use is just as critical as the purging technique itself.
Ultimately, mastering the inert air technique comes from understanding the fundamental goal: to actively and systematically displace the reactive atmosphere that surrounds us.
Summary Table:
| Key Aspect | Description |
|---|---|
| Purpose | To displace reactive air (O₂, H₂O) with non-reactive gas to protect sensitive materials. |
| Common Gases | Nitrogen (N₂) for cost-effectiveness, Argon (Ar) for maximum inertness. |
| Core Principle | Dilution via repeated purge cycles (3-5 times is standard) to reduce reactive gas to negligible levels. |
| Critical Factor | Maintain positive pressure with inert gas to prevent air from re-entering the system. |
| Common Setups | Glove boxes, Schlenk lines, and simple balloon techniques for varying levels of sensitivity. |
Need to create a reliable inert atmosphere for your lab work?
KINTEK specializes in providing the high-quality lab equipment and consumables you need to master the inert gas technique. Whether you require robust glove boxes, Schlenk line apparatus, or high-purity inert gases, we have the solutions to protect your sensitive materials from oxidation and hydrolysis.
Contact our experts today to discuss how we can help you achieve a contamination-free environment for your most critical experiments.
Visual Guide
Related Products
- High Performance Laboratory Freeze Dryer for Research and Development
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- CVD Diamond for Thermal Management Applications
People Also Ask
- What happens during the freezing phase of lyophilization? Master the Critical First Step for Product Integrity
- What is cool grinding technology? Unlock Efficient Milling for Heat-Sensitive Materials
- What are the benefits of cryogenic machining? Boost Tool Life, Finish, and Productivity
- Why is a freeze dryer considered essential in biological and chemical experiments? Preserve Sample Integrity for Accurate Results
- Why are conventional preservation methods less suitable for biological products? The Critical Risk to Efficacy and Safety










