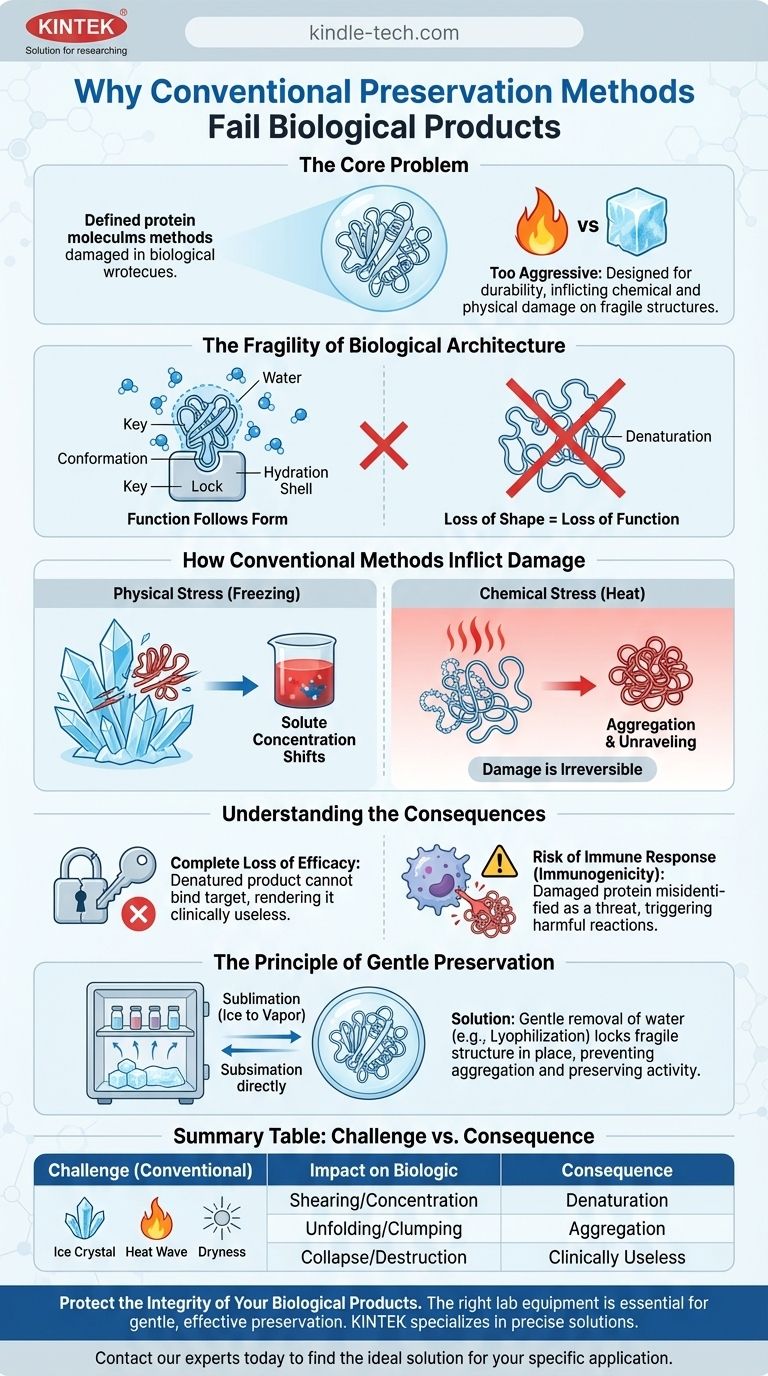
At their core, conventional preservation methods are simply too aggressive for biological products. Techniques like heat sterilization, chemical preservation, or simple freezing are designed for durability, but they inflict chemical and physical changes that irreparably damage the fragile, complex structures that make biologics work. This damage doesn't just reduce effectiveness; it can destroy it entirely.
The function of a biological product, like a protein or antibody, is dictated by its precise three-dimensional shape. Conventional preservation methods act as blunt instruments, altering or destroying this delicate architecture, rendering the product inactive and clinically useless.

The Fragility of Biological Architecture
Biological products are not simple chemical compounds; they are intricate molecular machines. Their therapeutic action depends entirely on maintaining their specific, complex structure.
Function Follows Form
Think of a therapeutic protein as a unique key designed for a specific lock in the body. Its function is absolutely dependent on its precise, folded three-dimensional shape, known as its conformation.
If this shape is altered, even slightly, the key no longer fits the lock. The product loses its ability to perform its intended function, a process called denaturation.
The Critical Role of Water
Water molecules are not just a solvent for biologics; they are an integral part of their structure. A hydration shell of water surrounds the molecule, helping to stabilize its delicate folds and maintain its correct shape.
Any preservation process must manage this water carefully. Simply removing it or freezing it incorrectly can cause the entire structure to collapse.
How Conventional Methods Inflict Damage
The forces used in traditional preservation are the very things that dismantle the architecture of a biological product.
Physical Stress: The Problem with Freezing
When water freezes, it forms ice crystals. These sharp, crystalline structures can physically shear, pierce, and unfold biological molecules, causing irreversible damage.
As ice forms, it also concentrates the remaining solutes (like salts) in the unfrozen water, drastically changing the solution's pH and ionic strength. This chemical shift places immense stress on the biologic, further contributing to its denaturation.
Chemical Stress: The Impact of Heat
Methods like pasteurization or autoclaving use high temperatures to sterilize products. This heat energy overwhelms the weak bonds holding a protein in its folded shape, causing it to unravel and clump together in a useless, aggregated mass.
This is the same process that happens when you cook an egg: the clear, liquid egg white (albumin protein) turns into a solid, white, and irreversibly denatured form.
Understanding the Consequences of Damage
The failure of a preservation method for a biologic goes beyond simple ineffectiveness; it can introduce significant patient risk.
Complete Loss of Efficacy
The most immediate consequence of structural damage is a total loss of therapeutic activity. The denatured protein or antibody can no longer bind to its target in the body.
This means the patient receives a product that has no clinical benefit, effectively rendering the treatment a failure.
Risk of Immune Response
A damaged or aggregated protein can be misidentified by the body's immune system as a foreign threat, like a virus or bacteria.
This can trigger an unwanted and potentially dangerous immune response, a phenomenon known as immunogenicity. Instead of helping the patient, the compromised product could cause harm.
The Principle of Gentle Preservation
The challenge with biologics is not merely to prevent decay but to do so while locking the molecule's fragile structure in place. This requires a fundamentally different approach.
- If your primary focus is maintaining biological activity: You must use methods like lyophilization (freeze-drying), which gently remove water by sublimation (turning ice directly into vapor), bypassing the damaging formation of large ice crystals.
- If your primary focus is ensuring patient safety: The preservation technique must be proven to prevent protein aggregation and conformational changes to eliminate the risk of an unintended immune response.
Ultimately, recognizing the profound difference between a simple chemical and a complex biologic is the foundation for creating safe and effective therapies.
Summary Table:
| Challenge with Conventional Methods | Impact on Biologic | Consequence |
|---|---|---|
| Physical Stress (Freezing) | Ice crystals shear and pierce molecules; solute concentration shifts. | Irreversible structural damage (denaturation). |
| Chemical Stress (Heat) | Weak molecular bonds break, causing proteins to unfold and clump. | Complete loss of therapeutic activity (aggregation). |
| Improper Water Removal | Disruption of the essential hydration shell stabilizing the 3D structure. | Molecule collapses, becoming clinically useless. |
Protect the integrity of your delicate biological products. The right lab equipment is essential for gentle, effective preservation techniques like lyophilization. KINTEK specializes in providing the precise, reliable lab equipment and consumables your laboratory needs to ensure biologic efficacy and patient safety. Don't let improper preservation compromise your research or therapies. Contact our experts today to find the ideal solution for your specific application.
Visual Guide
Related Products
- High Performance Laboratory Freeze Dryer
- High Performance Laboratory Freeze Dryer for Research and Development
- Benchtop Laboratory Freeze Dryer for Lab Use
- Low-Temperature Water-Cooled Touchscreen Vibratory Ultrafine Pulverizer
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
People Also Ask
- What is cryogenic grinding of cardamom? Preserve Flavor, Aroma & Color with Extreme Cold
- Why are plate temperature uniformity and flatness important in a freeze dryer? Ensure Product Quality and Process Efficiency
- Why is a freeze dryer considered essential in biological and chemical experiments? Preserve Sample Integrity for Accurate Results
- What process advantages are offered by integrating a cryogenic cooling device during HPT? Achieve Ultimate Grain Refinement
- What happens during the freezing phase of lyophilization? Master the Critical First Step for Product Integrity



















