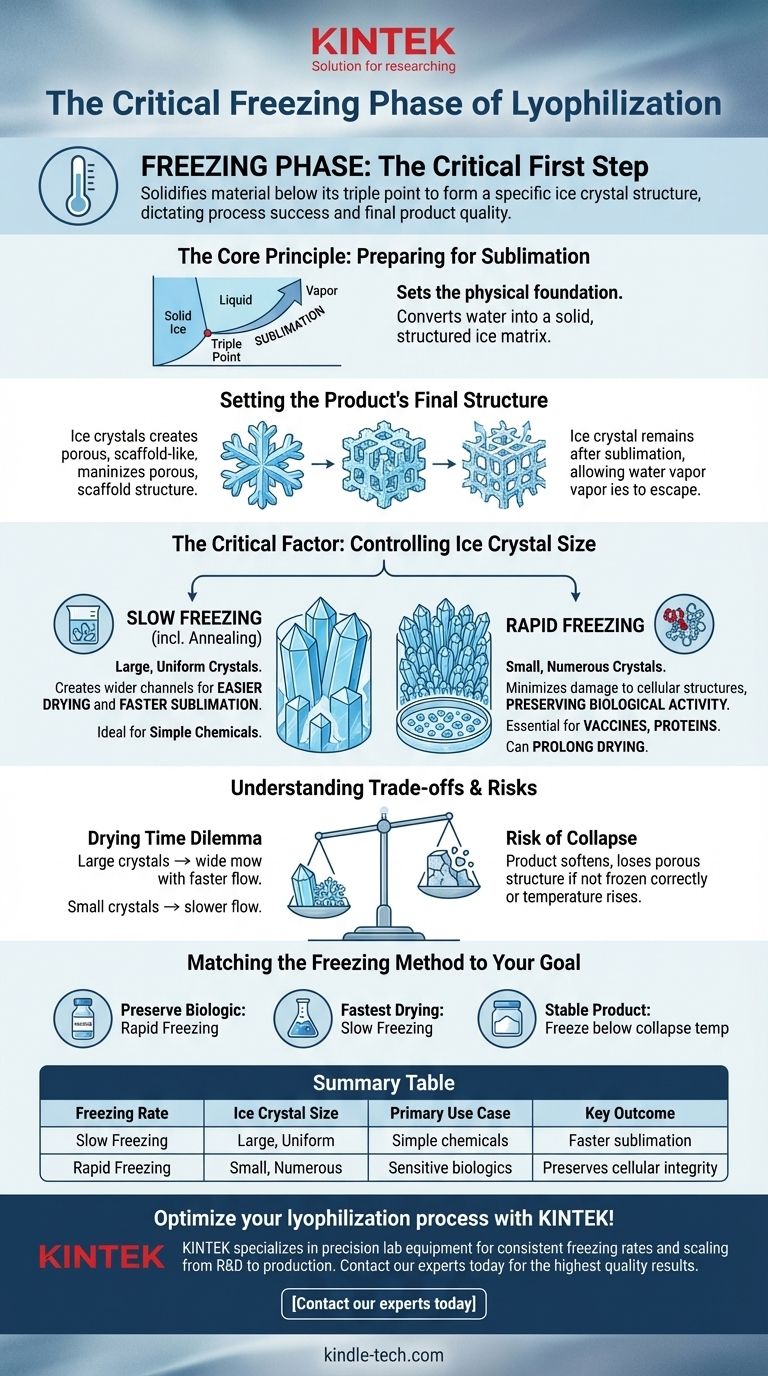
In lyophilization, the freezing phase is the critical first step where the material is solidified at a temperature below its triple point. This process is not merely about making the product cold; it is about deliberately forming a specific ice crystal structure that dictates the success of the entire drying process and the quality of the final product.
The core purpose of the freezing phase is to convert all water into a solid, structured ice matrix. The rate of this freezing determines the size of the ice crystals, which in turn controls both the final product's physical structure and the efficiency of the subsequent drying step.

The Core Principle: Preparing for Sublimation
The initial freeze sets the physical foundation for the entire lyophilization process. If this stage is handled incorrectly, no amount of optimization in later stages can recover the product.
Freezing Below the Triple Point
The triple point is the unique combination of temperature and pressure at which a substance's solid, liquid, and gaseous phases can coexist.
By freezing the material well below this point, we ensure that when a vacuum is applied later, the solid ice will bypass the liquid phase and turn directly into vapor. This direct solid-to-gas transition is called sublimation, and it is the central mechanism of freeze-drying.
Setting the Product's Final Structure
During freezing, the ice crystals that form create a porous, scaffold-like structure within the product.
When this ice is later removed via sublimation, these pores remain. This network of channels is essential for allowing water vapor from deep inside the product to escape during the drying phase.
The Critical Factor: Controlling Ice Crystal Size
The speed at which you freeze the material directly controls the size of the ice crystals, which has profound implications for the final product.
Slow Freezing for Easier Drying
A slow freezing process, sometimes including a temperature-cycling step called annealing, allows water molecules more time to migrate and form large, uniform ice crystals.
These large crystals create wider, more interconnected channels in the dried product. This structure makes it much easier for water vapor to escape, which can significantly speed up the sublimation phase.
Rapid Freezing for Biological Preservation
For sensitive biological materials like vaccines, proteins, or antibodies, large ice crystals are destructive. Their sharp edges can puncture and destroy cell walls and membranes, negating the entire purpose of preservation.
Rapid freezing creates vast numbers of very small ice crystals. This minimizes physical damage to cellular structures and is essential for preserving the biological activity of the final product.
Understanding the Trade-offs and Risks
The choice of freezing rate is a balance between preserving the product's integrity and optimizing the process efficiency.
The Drying Time Dilemma
The structure created during freezing directly impacts drying time.
Large ice crystals from slow freezing result in a more porous cake and faster sublimation. In contrast, the small crystals from rapid freezing create a denser product with smaller pores, which increases resistance to vapor flow and can significantly prolong the drying phase.
The Risk of Collapse
If the product is not frozen correctly or if its temperature rises above a critical point during drying, a failure known as collapse can occur.
Collapse happens when the product softens and can no longer support its own porous structure. This leads to incomplete drying, poor solubility, and a complete loss of the product's intended form. A proper freezing protocol establishes a robust structure that can withstand the rigors of sublimation.
Matching the Freezing Method to Your Goal
Your specific objective dictates the optimal freezing strategy.
- If your primary focus is preserving biological activity (e.g., vaccines, enzymes): Choose rapid freezing to create small ice crystals and prevent cellular damage, even if it extends drying time.
- If your primary focus is the fastest possible drying time for a robust, simple chemical: Use slow freezing or an annealing step to generate large ice crystals that facilitate rapid sublimation.
- If your primary focus is a stable, easily re-dissolvable final product: Ensure you freeze well below the material's critical collapse temperature to build a robust structural foundation.
Mastering the freezing phase is the key to unlocking the full potential of lyophilization, ensuring both product integrity and process efficiency.
Summary Table:
| Freezing Rate | Ice Crystal Size | Primary Use Case | Key Outcome |
|---|---|---|---|
| Slow Freezing | Large, Uniform | Simple chemicals, faster drying | Faster sublimation, porous structure |
| Rapid Freezing | Small, Numerous | Sensitive biologics (vaccines, proteins) | Preserves cellular integrity, longer drying time |
Optimize your lyophilization process with KINTEK!
The freezing phase is the foundation of successful freeze-drying. Whether you are developing vaccines, preserving proteins, or processing chemicals, the right equipment is critical for controlling ice crystal formation and ensuring product quality.
KINTEK specializes in precision lab equipment for all your lyophilization needs. Our solutions help you achieve consistent freezing rates, maintain precise temperatures, and scale your process from R&D to production.
Contact our experts today to discuss how we can support your specific application and ensure your lyophilization process delivers the highest quality results.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- High Performance Laboratory Freeze Dryer for Research and Development
- High Performance Laboratory Freeze Dryer
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Low-Temperature Water-Cooled Touchscreen Vibratory Ultrafine Pulverizer
People Also Ask
- Why is a laboratory freeze-drying system essential for fermentation biomass? Preserve Sample Integrity for Analysis
- What is the function of Freeze-thaw Equipment in Au-(PNiPAAm/PVA) hydrogel? Achieve High-Speed Photothermal Actuation
- Why is a freeze dryer preferred for reduced graphene oxide (Hh-RGO) powders? Preserve Nano-Structure and Performance
- Why is a freeze dryer preferred for drying nickel nanoparticle precursors? Prevent Hard Agglomeration Now
- What are the advantages of using freeze drying for phase change materials with biopolymer shells? Optimize Stability



















