In essence, freeze drying is a sophisticated three-stage dehydration process. It works by first freezing a product, then placing it in a deep vacuum which causes the frozen water to turn directly into vapor, a process called sublimation. This method is uniquely gentle, removing moisture while perfectly preserving the product's original structure, chemical composition, and biological activity.
The critical difference between freeze drying and all other dehydration methods is that it bypasses the liquid water phase. This prevents the shrinking and structural damage caused by conventional evaporation, making it the gold standard for preserving delicate materials.

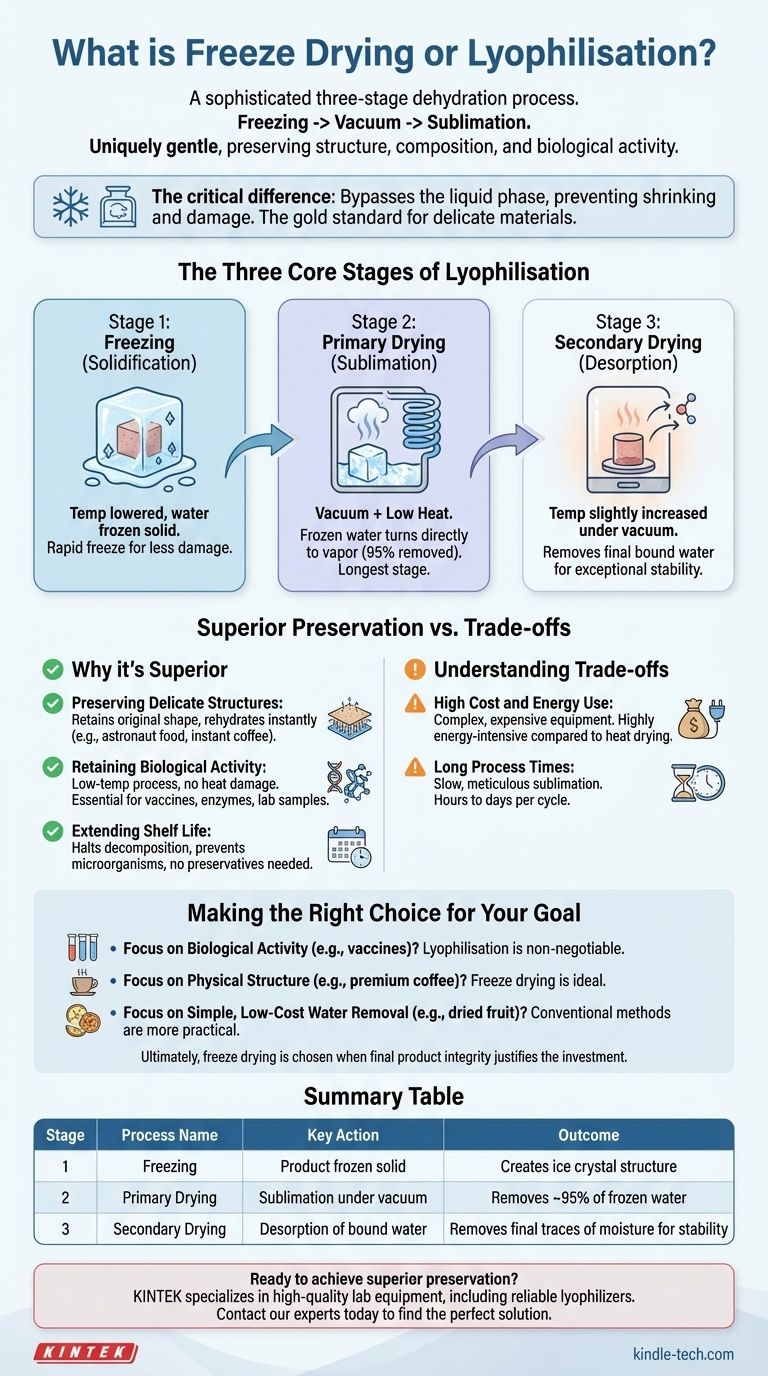
The Three Core Stages of Lyophilisation
The entire process, whether called freeze drying or lyophilisation, is managed by a machine known as a lyophilizer. It meticulously controls temperature and pressure to navigate three distinct stages.
Stage 1: Freezing (Solidification)
The first step is to lower the product's temperature until all the water within it is frozen solid.
This is a critical phase because the way the water freezes determines the final structure of the product. A rapid freeze creates small ice crystals, which cause less damage to the cellular walls.
Stage 2: Primary Drying (Sublimation)
With the product solidly frozen, a powerful vacuum pump is activated, dramatically lowering the pressure inside the chamber.
This combination of low pressure and a small amount of added heat gives the frozen water molecules enough energy to transform directly into a gas or vapor. This vapor is then drawn away from the product and collected on an extremely cold condenser coil, turning back into ice.
This is the longest stage of the process, removing up to 95% of the water.
Stage 3: Secondary Drying (Desorption)
The final stage is designed to remove the last traces of unfrozen water molecules that are chemically bound to the product.
By slightly increasing the temperature while maintaining the vacuum, these final water molecules are released. This step ensures the final product is exceptionally dry and stable for long-term storage.
Why This Method is a Superior Form of Preservation
The complexity of freeze drying is justified by its unparalleled results, particularly for sensitive materials.
Preserving Delicate Structures
Because the water turns directly from a solid to a gas, the product's underlying structure is left intact. This is why freeze-dried food, like astronaut ice cream or instant coffee, retains its original shape and rehydrates almost instantly.
Retaining Biological Activity
Conventional drying uses heat, which can destroy or "denature" sensitive proteins, enzymes, and other biological compounds.
Lyophilisation is a low-temperature process, making it the essential method for preserving vaccines, antibodies, pharmaceuticals, and laboratory samples where biological function must be maintained.
Extending Shelf Life
By removing virtually all water, freeze drying halts decomposition and prevents the growth of microorganisms. This gives products an incredibly long and stable shelf life without the need for preservatives.
Understanding the Trade-offs
While highly effective, lyophilisation is not the solution for every scenario. It involves significant trade-offs that make it a specialized technique.
High Cost and Energy Use
Lyophilizers are complex, expensive pieces of equipment. The process of maintaining deep vacuums and extremely low temperatures for extended periods is also highly energy-intensive compared to simple heat-based dehydration.
Long Process Times
Sublimation is a slow, meticulous process. A single cycle of freeze drying can take anywhere from several hours to multiple days, depending on the product. This makes it a much slower method than conventional oven or air drying.
Making the Right Choice for Your Goal
Selecting a dehydration method depends entirely on the nature of your product and your preservation goals.
- If your primary focus is preserving biological activity (e.g., vaccines, enzymes): Lyophilisation is the non-negotiable standard because its low-temperature process prevents heat damage.
- If your primary focus is maintaining physical structure (e.g., premium coffee, emergency food): Freeze drying is the ideal choice for a product that rehydrates quickly while retaining its original texture and shape.
- If your primary focus is simple, low-cost water removal (e.g., jerky, dried fruit): Conventional methods like air or heat drying are far more practical, economical, and efficient.
Ultimately, freeze drying is a powerful tool chosen when the integrity of the final product justifies the investment in time and energy.
Summary Table:
| Stage | Process Name | Key Action | Outcome |
|---|---|---|---|
| 1 | Freezing | Product is frozen solid | Creates ice crystal structure |
| 2 | Primary Drying | Sublimation under vacuum | Removes ~95% of frozen water |
| 3 | Secondary Drying | Desorption of bound water | Removes final traces of moisture for stability |
Ready to achieve superior preservation for your sensitive materials? The freeze drying process is essential for labs and manufacturers who need to maintain the structural integrity and biological activity of pharmaceuticals, vaccines, food, and other delicate products. KINTEK specializes in high-quality lab equipment, including reliable lyophilizers, to meet your precise dehydration needs. Contact our experts today to find the perfect solution for your laboratory.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Desktop Fast High Pressure Laboratory Autoclave Sterilizer 16L 24L for Lab Use
- Small Lab Rubber Calendering Machine
- Single Punch Tablet Press Machine and Mass Production Rotary Tablet Punching Machine for TDP
People Also Ask
- What are the advantages of using freeze drying for phase change materials with biopolymer shells? Optimize Stability
- What role does a laboratory freeze dryer play in the synthesis of graphene-based electrocatalysts? Preserve 3D Structures
- Why is a freeze dryer preferred for reduced graphene oxide (Hh-RGO) powders? Preserve Nano-Structure and Performance
- What is the function of a freeze dryer in the ice-templating process? Preserving Aligned Pore Scaffolds for LAGP
- Why is a laboratory vacuum freeze dryer essential for plant extracts? Preserve Bioactivity & Structure



















