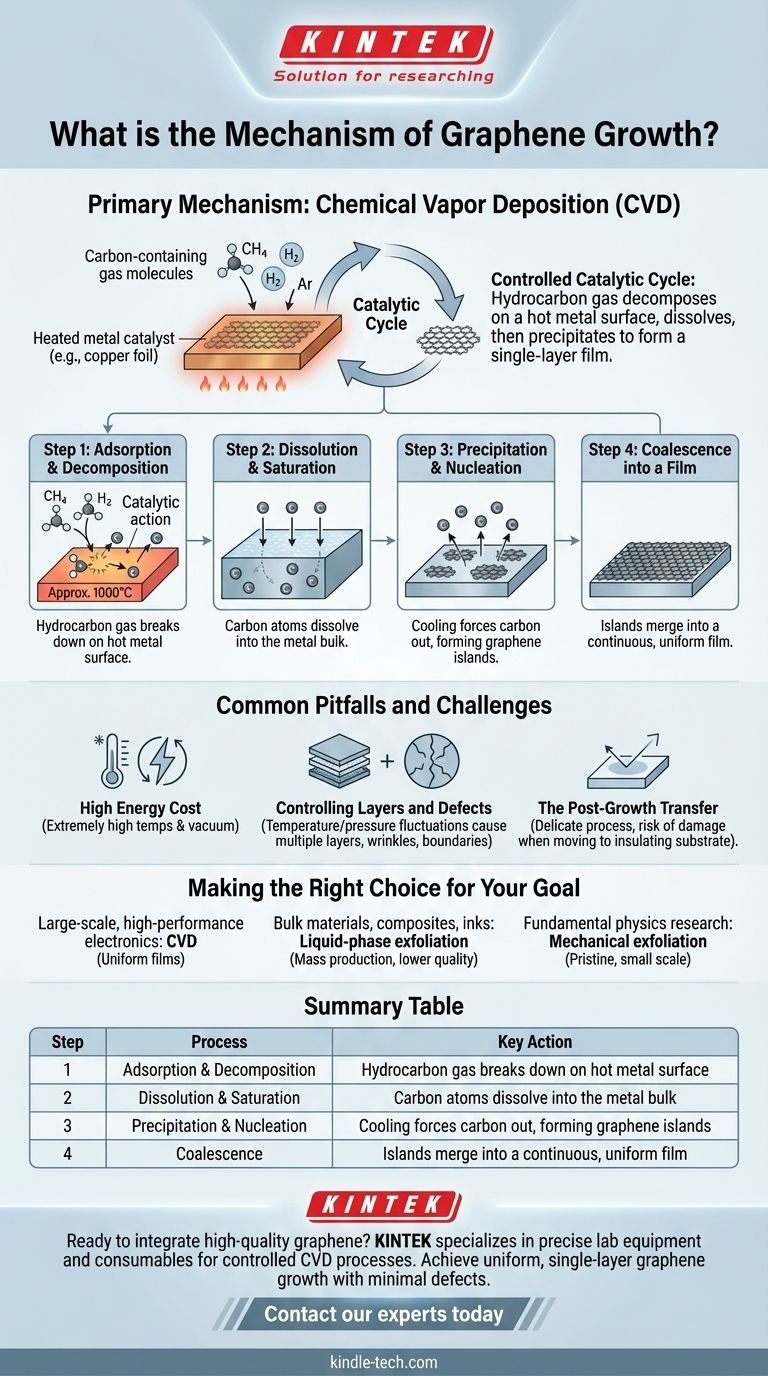
The primary mechanism for growing high-quality graphene is a process called Chemical Vapor Deposition (CVD). This method involves exposing a heated metal catalyst, typically a transition metal like copper or nickel, to a carbon-containing gas. The high temperature causes the gas to decompose, depositing carbon atoms that then self-assemble into a single, continuous atomic layer on the metal's surface.
The core of graphene growth via CVD is not simply a deposition, but a controlled catalytic cycle. It relies on a hydrocarbon gas decomposing on a hot metal surface, with carbon atoms first dissolving into the metal and then precipitating out upon cooling to form a high-quality, single-layer film.

Deconstructing the CVD Process for Graphene
The CVD process can be understood as a sequence of controlled physical and chemical steps. Each stage is critical for forming a uniform, single-atomic-layer film over a large area.
### The Role of the Catalyst Substrate
The entire process begins with a substrate, which is almost always a transition metal. These metals are chosen because they act as both the surface for growth and a catalyst that facilitates the chemical reactions.
Their catalytic properties lower the energy required to break down the carbon-source gas molecules into individual carbon atoms.
### The Carbon Source: Hydrocarbon Gases
The source of carbon is a hydrocarbon gas, most commonly methane (CH₄). This gas is mixed with other gases, like hydrogen and argon, and flowed into a high-temperature furnace where the catalyst substrate is waiting.
### Step 1: Adsorption and Decomposition
At very high temperatures (often around 1000°C), the hydrocarbon gas molecules land on the hot metal surface (adsorption). The catalytic nature of the metal and the intense heat break the chemical bonds in the gas, releasing individual carbon atoms.
### Step 2: Dissolution and Saturation
Once freed, these carbon atoms don't immediately form graphene. Instead, they dissolve into the bulk of the metal foil, much like sugar dissolves in hot water. This process continues until the metal becomes saturated with carbon atoms.
### Step 3: Precipitation and Nucleation
This is the most critical step. As the furnace is cooled, the solubility of carbon in the metal decreases significantly. The metal can no longer hold all the dissolved carbon, forcing the atoms to come back out, or precipitate, onto the surface.
These precipitating carbon atoms begin to bond with each other, forming small, island-like patches of graphene known as nucleation sites.
### Step 4: Coalescence into a Film
As cooling continues, these islands grow larger and eventually merge together (coalesce), forming a continuous and uniform sheet of single-layer graphene that covers the entire surface of the metal substrate.
Common Pitfalls and Challenges
While CVD is the most promising technique for high-quality, large-area graphene, it is not without its difficulties. Understanding these challenges is key to optimizing the process.
### The High Energy Cost
The process requires extremely high temperatures and often relies on high-vacuum systems. This makes the equipment complex and the growth process itself highly energy-intensive.
### Controlling Layers and Defects
The growth is difficult to control perfectly. Minor fluctuations in temperature, pressure, or gas flow can lead to the formation of multiple graphene layers instead of a single one. It can also create defects like wrinkles or grain boundaries where the graphene islands meet imperfectly.
### The Post-Growth Transfer
Graphene grown via CVD is on a metal substrate, which is not useful for most electronic applications. It must be carefully transferred to an insulating substrate, like silicon or glass. This transfer process is delicate and can easily introduce tears, wrinkles, and contamination, degrading the material's quality.
Making the Right Choice for Your Goal
The best method for producing graphene depends entirely on the intended application and desired balance of quality, quantity, and cost.
- If your primary focus is large-scale, high-performance electronics: CVD is the only viable mechanism, as it produces the large, high-quality, and uniform films required.
- If your primary focus is bulk materials like composites or conductive inks: Liquid-phase exfoliation is a more suitable choice for mass production, though you must accept a trade-off in lower electrical quality.
- If your primary focus is fundamental physics research on pristine samples: Mechanical exfoliation remains a key method for producing the highest-quality, defect-free graphene flakes, although only on a very small scale.
Ultimately, mastering the complex interplay of catalyst, temperature, and atmosphere in the growth mechanism is the key to unlocking graphene's full technological potential.
Summary Table:
| Step | Process | Key Action |
|---|---|---|
| 1 | Adsorption & Decomposition | Hydrocarbon gas breaks down on hot metal surface |
| 2 | Dissolution & Saturation | Carbon atoms dissolve into the metal bulk |
| 3 | Precipitation & Nucleation | Cooling forces carbon out, forming graphene islands |
| 4 | Coalescence | Islands merge into a continuous, uniform film |
Ready to integrate high-quality graphene into your research or production? KINTEK specializes in providing the precise lab equipment and consumables needed for controlled CVD processes. Our expertise ensures you achieve uniform, single-layer graphene growth with minimal defects. Contact our experts today to discuss how we can support your laboratory's advanced material synthesis goals.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- What color diamonds are CVD? Understanding the Process from Brown Tint to Colorless Beauty
- What is the vapor phase deposition technique? A Guide to PVD & CVD Thin-Film Coating Methods
- How does PECVD work? Enable Low-Temperature, High-Quality Thin Film Deposition
- What is PECVD in semiconductor? Enable Low-Temperature Thin Film Deposition for ICs
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application



















