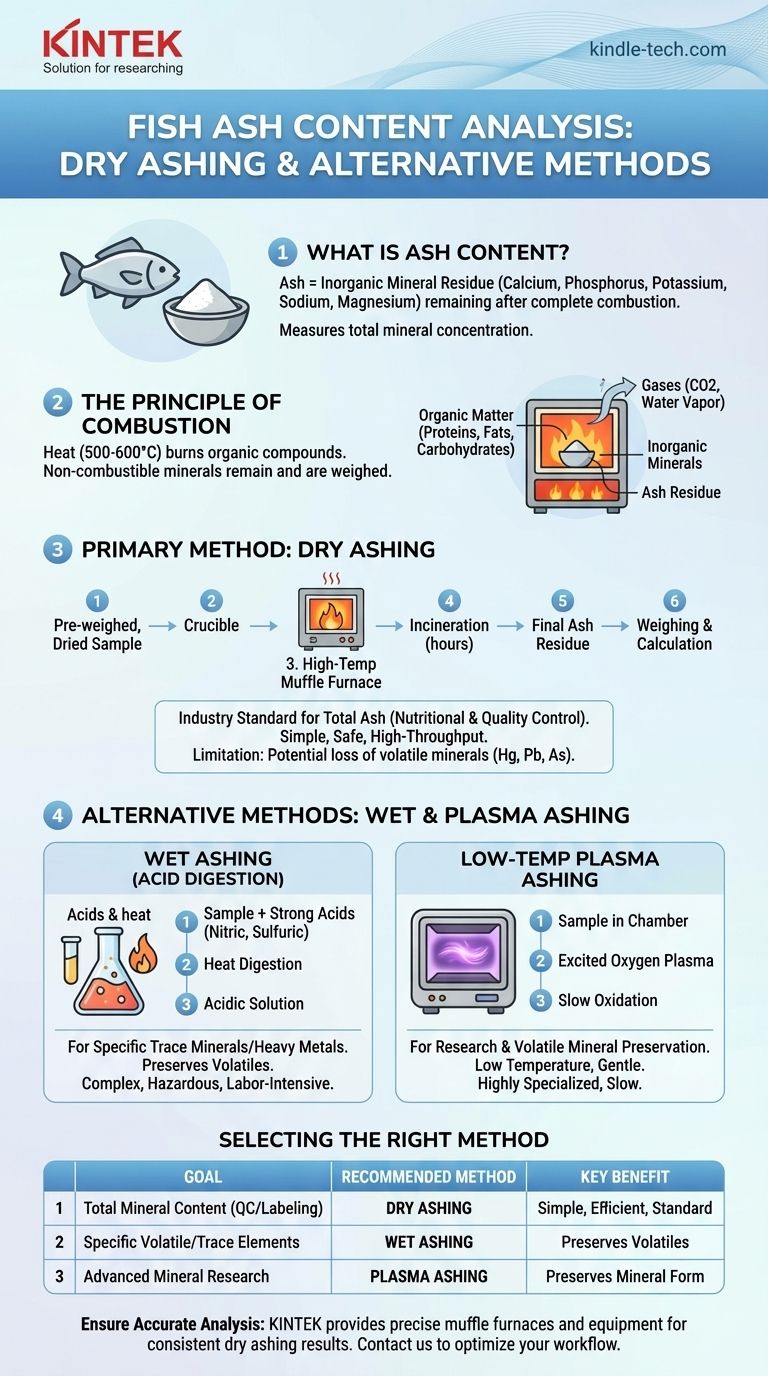
The primary method used to determine the ash content of fish and other food products is called dry ashing. This process involves the complete combustion of a sample at a very high temperature in a muffle furnace, which burns away all organic matter and leaves behind only the inorganic mineral residue, known as ash. While other methods like wet ashing exist, dry ashing is the standard for determining total mineral content for nutritional and quality control purposes.
The core purpose of measuring ash is to quantify the total non-combustible mineral content in a product. The chosen method is simply a controlled process of incineration designed to isolate these inorganic materials from the organic components like protein, fat, and carbohydrates.

What "Ash Content" Truly Represents
A Measure of Total Mineral Content
The term "ash" is the technical name for the inorganic residue that remains after a food sample is completely incinerated. This residue consists of the essential minerals present in the fish, such as calcium, phosphorus, potassium, sodium, and magnesium, in the form of stable oxides.
Ash content is a fundamental measure of the nutritional value of food, serving as a direct indicator of its total mineral concentration.
The Principle of Complete Combustion
The analysis works on a simple principle: organic compounds combust, while inorganic minerals do not.
By heating a sample to a high temperature (typically 500-600°C), all the organic components—proteins, fats, and carbohydrates—are oxidized and converted into gases like carbon dioxide and water vapor. The material that remains is the non-combustible mineral portion, which is then weighed.
A Breakdown of the Common Methods
Dry Ashing (The Standard Method)
This is the most widely used technique for routine analysis. A pre-weighed, dried sample is placed in a crucible and heated in a muffle furnace at a high, controlled temperature.
The sample is incinerated for several hours until all organic matter is gone, leaving a fine, gray or white ash. The final weight of this ash allows for the calculation of the total ash content percentage.
Wet Ashing (For Specific Mineral Analysis)
Wet ashing, or acid digestion, uses a combination of strong acids (like nitric and sulfuric acid) and heat to oxidize and break down the organic matrix.
This method is not typically used for determining total ash content. Instead, it is a sample preparation technique for analyzing specific trace minerals or heavy metals, as the resulting acidic solution is ideal for analysis by methods like atomic absorption spectroscopy (AAS).
Low-Temperature Plasma Ashing
This is a more specialized and gentler method. It uses excited oxygen gas (plasma) at lower temperatures to slowly oxidize the organic matter.
It is primarily used in research settings where preventing the loss of volatile minerals is critical or when the chemical form of the mineral needs to be preserved for further study.
Understanding the Trade-offs
Why Dry Ashing is So Common
Dry ashing is the industry standard for total ash determination because it is relatively simple, safe, and allows for the simultaneous processing of many samples. It requires minimal hands-on time and does not involve handling highly corrosive acids.
The Limitation of High Temperatures
The main drawback of dry ashing is the potential loss of volatile minerals. Elements like mercury, lead, and arsenic, as well as some forms of chlorine and sulfur, can vaporize at the high temperatures used in a muffle furnace.
This makes dry ashing unsuitable if the goal is to accurately quantify these specific volatile elements.
The Complexity of Wet Ashing
While wet ashing preserves volatile minerals, it is a more complex, hazardous, and labor-intensive process. It requires specialized ventilation (fume hoods) to handle the corrosive acid fumes and demands constant operator attention.
For this reason, it is reserved for applications where the analysis of specific trace elements, not total ash, is the primary objective.
Selecting the Right Method for Your Goal
Choosing the correct method depends entirely on the question you need to answer.
- If your primary focus is nutritional labeling or general quality control: Use the standard dry ashing method to efficiently determine the total mineral content.
- If your primary focus is analyzing for specific volatile minerals or heavy metals: Use wet ashing to prepare the sample for further instrumental analysis.
- If your primary focus is advanced research on mineral states: Consider low-temperature plasma ashing, though it is highly specialized and less common.
Ultimately, understanding your analytical goal is the key to choosing the correct ashing technique for your work.
Summary Table:
| Method | Primary Use | Key Advantage | Key Limitation |
|---|---|---|---|
| Dry Ashing | Total mineral content (Nutritional/QC) | Simple, safe, high-throughput | Loss of volatile minerals |
| Wet Ashing | Specific trace/heavy metal analysis | Preserves volatile minerals | Complex, hazardous, uses strong acids |
| Plasma Ashing | Research on mineral states | Low temperature, preserves mineral form | Specialized, slow, less common |
Ensure Accurate Mineral Analysis in Your Lab
Choosing the right ashing method is critical for reliable nutritional labeling and quality control. KINTEK specializes in providing the precise lab equipment, including high-quality muffle furnaces and consumables, that your laboratory needs for consistent and accurate dry ashing results.
Let our experts help you optimize your workflow. Contact KINTEK today to discuss your specific application and find the perfect solution for your laboratory's needs.
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What precautions should you take while using a muffle furnace? Ensure Safe High-Temperature Processing in Your Lab
- How do you cool down a muffle furnace? Ensure Longevity and Safety with the Correct Procedure
- What is the working principle of a muffle furnace? Achieve Precise, Contamination-Free Heating
- What is the operating temperature of the muffle furnace? Find Your Ideal Range for Lab Success
- What is the working principle of laboratory muffle furnace? Achieve Contamination-Free High-Temperature Processing



















