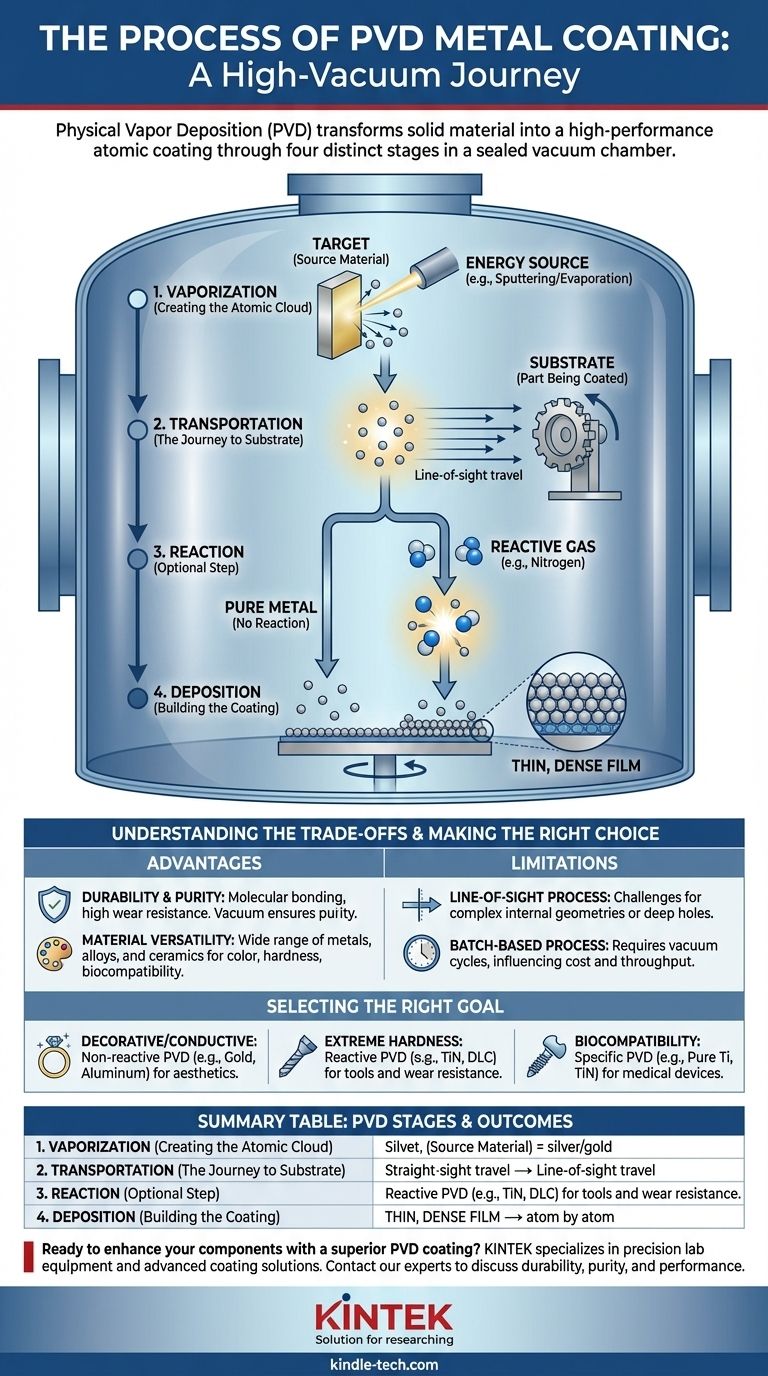
In essence, Physical Vapor Deposition (PVD) for metal is a high-vacuum process that transforms a solid source material, known as the "target," into a vapor. This vapor then travels through the vacuum chamber and condenses onto the surface of a part, or "substrate," building a new, high-performance coating one atom at a time. The entire process generally unfolds across four distinct stages: vaporization, transportation, reaction, and deposition.
The core principle of PVD is the physical transfer of material from a source to a surface without altering the material's fundamental chemistry unless a reaction is intentionally introduced. Understanding that this "reaction" step is optional is the key to grasping PVD's versatility for creating both pure metal finishes and ultra-hard ceramic coatings.

Deconstructing the PVD Process
To truly understand how PVD works, it's best to visualize it as a controlled, four-stage journey that takes place inside a sealed chamber. Each stage plays a critical role in determining the final properties of the coating.
The Foundation: A High-Vacuum Environment
Before any coating can begin, air and other gases are pumped out of the PVD chamber to create a near-perfect vacuum. This is non-negotiable.
This high-vacuum environment is critical because it prevents the metal vapor from reacting with airborne contaminants like oxygen or water, ensuring a pure coating. It also allows the vaporized atoms to travel freely from the source to the part without colliding with air molecules.
Stage 1: Vaporization (Creating the Atomic Cloud)
This is the step where the solid coating material is converted into a gas. A high-energy source is directed at the target (the block of pure source material).
The most common methods are sputtering, which bombards the target with energetic ions to physically knock atoms loose, or thermal evaporation, which uses heat to boil the material into a vapor. The result is a cloud of gaseous source material.
Stage 2: Transportation (The Journey to the Substrate)
Once vaporized, the atoms or molecules travel through the vacuum chamber from the target towards the substrate (the part being coated).
This is typically a "line-of-sight" journey. The vapor particles travel in a straight line until they strike a surface, which is why parts are often rotated on complex fixtures to ensure even coverage.
Stage 3: Reaction (The Optional but Critical Step)
This stage is what defines the two major categories of PVD. It may or may not occur, depending on the desired outcome.
If the goal is a pure metal coating (like gold or aluminum), this step is skipped. However, if the goal is an extremely hard ceramic coating, a controlled amount of a reactive gas (like nitrogen or oxygen) is introduced into the chamber. The metal atoms react with this gas to form a new compound, such as titanium nitride or chromium oxide.
Stage 4: Deposition (Building the Coating Atom by Atom)
In the final stage, the metal vapor (or newly formed compound vapor) reaches the substrate and condenses onto its surface.
Because the substrate is often at a lower temperature, the vapor rapidly solidifies, creating a very dense, thin, and highly adherent film. This atomic-level deposition is what gives PVD coatings their superior strength and uniformity.
Understanding the Trade-offs
PVD is a powerful technology, but it's essential to recognize its specific advantages and limitations to determine if it is the right solution for your application.
Advantage: Durability and Purity
PVD coatings are molecularly bonded to the substrate, making them incredibly hard and resistant to wear, corrosion, and abrasion. The vacuum process ensures a level of purity that is difficult to achieve with other methods like electroplating.
Advantage: Material Versatility
The process allows for the deposition of pure metals, complex alloys, and extremely hard ceramic compounds. This gives engineers and designers a vast palette of materials to choose from for properties ranging from color and conductivity to hardness and biocompatibility.
Limitation: Line-of-Sight Process
Because the vapor travels in a straight line, coating complex internal geometries or deep, narrow holes can be challenging. Achieving uniform thickness requires careful part orientation and often complex rotating fixtures within the chamber.
Limitation: A Batch-Based Process
PVD is not a continuous-flow process. Parts must be loaded into a chamber, the vacuum must be created, the process run, and then the chamber vented to remove the parts. This batch nature can influence cost and throughput compared to other finishing methods.
Making the Right Choice for Your Goal
Your end goal will determine which variation of the PVD process is most appropriate.
- If your primary focus is a decorative or conductive pure metal finish: Non-reactive PVD is the correct choice, where the "Reaction" step is omitted to deposit materials like gold, aluminum, or chromium directly.
- If your primary focus is extreme hardness and wear resistance: Reactive PVD is necessary to form hard ceramic compounds like Titanium Nitride (TiN), Chromium Nitride (CrN), or Diamond-Like Carbon (DLC) on tools and components.
- If your primary focus is biocompatibility for medical devices: Specific non-reactive or reactive PVD processes are chosen to deposit inert materials like pure Titanium or Titanium Nitride (TiN), which are safe for contact with the human body.
By understanding these fundamental stages, you can move beyond simply specifying "PVD" and begin to intentionally select the right material and process to achieve your desired performance.
Summary Table:
| PVD Stage | Key Action | Outcome |
|---|---|---|
| 1. Vaporization | Solid target material is converted to vapor via sputtering or evaporation. | Creates a cloud of source atoms. |
| 2. Transportation | Vapor travels in a straight line through the vacuum chamber. | Atoms move from source to substrate. |
| 3. Reaction (Optional) | Vapor reacts with a gas (e.g., Nitrogen) to form a compound. | Creates ultra-hard ceramic coatings (e.g., TiN). |
| 4. Deposition | Vapor condenses and bonds to the substrate surface. | Forms a dense, thin, and highly adherent film. |
Ready to enhance your components with a superior PVD coating?
KINTEK specializes in precision lab equipment and consumables for advanced coating processes. Whether you need to develop durable tool coatings, biocompatible medical device finishes, or decorative metal layers, our expertise ensures you achieve the exact material properties you require.
Contact our experts today to discuss how our PVD solutions can bring durability, purity, and performance to your laboratory or manufacturing process.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
- Molybdenum Tungsten Tantalum Special Shape Evaporation Boat
People Also Ask
- What are the applications of PECVD? Essential for Semiconductors, MEMS, and Solar Cells
- What are the advantages of PECVD? Enable Low-Temperature, High-Quality Thin-Film Deposition
- Why does PECVD commonly use RF power input? For Precise Low-Temperature Thin Film Deposition
- What is plasma activated chemical vapour deposition method? A Low-Temperature Solution for Advanced Coatings
- What is an example of PECVD? RF-PECVD for High-Quality Thin Film Deposition



















