At its core, sintering is a thermal process that transforms a compacted powder into a dense, solid object. It uses high temperatures—but crucially, temperatures below the melting point of the primary material—to fuse individual particles together. This is accomplished through atomic diffusion, where atoms migrate across the boundaries of the particles, creating strong, permanent bonds.
The fundamental principle of sintering is not melting, but solid-state diffusion. By heating a material enough to make its atoms mobile, the process eliminates the empty spaces between powder particles, creating a solid, unified mass with properties approaching that of a fully melted and cast material.
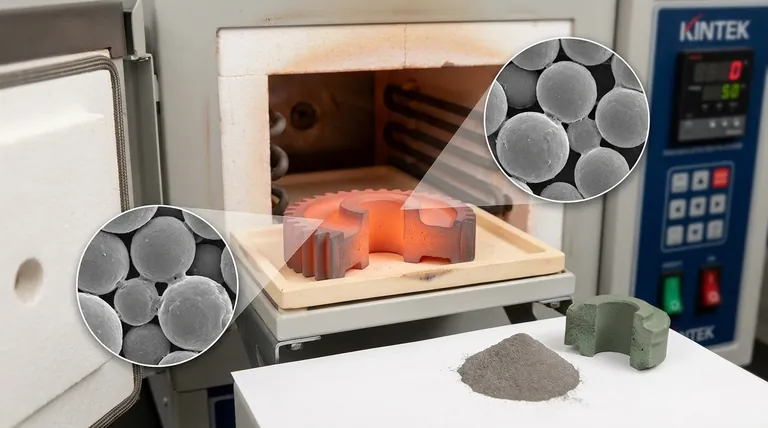
The Sintering Process: A Step-by-Step Breakdown
Sintering is a multi-stage process that requires precise control over composition, shape, and temperature to achieve the desired final properties. Each step plays a critical role in the success of the final part.
Step 1: Forming the "Green Part"
The process begins by creating an initial, fragile shape known as a "green part" or compact. A primary powder (metal or ceramic) is mixed with a temporary binding agent, such as wax, polymer, or water.
This mixture is then compacted into the desired shape. This is commonly done by pressing it into a die or mold, but other methods like 3D printing can also be used. The binder's only job is to hold the powder particles together until the heating begins.
Step 2: Binder Burn-off (Debinding)
The green part is placed into a sintering furnace. The temperature is first raised to a relatively low level.
During this initial heating stage, the temporary binding agent is systematically burned off or evaporated. This step must be done carefully to prevent the part from cracking or deforming as the binder exits.
Step 3: High-Temperature Fusion
With the binder removed, the furnace temperature is increased significantly, approaching (but not reaching) the melting point of the primary material.
At this high temperature, atoms at the contact points between particles gain enough energy to move and diffuse across the particle boundaries. This phenomenon, known as necking, forms bridges between particles. As these bridges grow, they pull the particle centers closer together, systematically reducing porosity and increasing the part's density.
Step 4: Controlled Cooling
After holding the part at the sintering temperature for a specified time, it is cooled in a controlled manner.
This cooling process allows the newly formed bonds to solidify and the material's final microstructure to set. The resulting object is a single, solid mass with properties vastly different from the original powder.
Key Mechanisms and Variations
While the steps are consistent, the underlying science explains why sintering is so effective and versatile. Understanding these mechanisms reveals the true power of the process.
The Role of Atomic Diffusion
Sintering works because heat gives atoms kinetic energy. Even in a solid state, atoms near the surface of each powder grain become mobile enough to "jump" over to a neighboring grain.
This migration of atoms builds strong metallic or ceramic bonds at the contact points, effectively welding the particles together on a microscopic scale without ever liquefying the bulk material.
Porosity Reduction and Densification
The primary goal of sintering is to increase density. As atomic diffusion forms and enlarges the "necks" between particles, the particles are pulled into the empty spaces (pores) that once existed between them.
This action dramatically reduces the part's internal porosity and causes it to shrink. The result is a much stronger, more solid component.
The Liquid Phase Sintering (LPS) Variant
In some cases, a second powder with a lower melting point is added to the primary mix.
During heating, this additive melts and becomes a liquid, flowing into the pores between the solid primary particles. The liquid acts as a catalyst, accelerating the diffusion and rearrangement of the solid particles, leading to faster and more complete densification before the part is cooled.
Understanding the Trade-offs
Sintering is a powerful technique, but it comes with specific considerations that are critical for successful implementation.
Inherent Porosity
While sintering significantly reduces voids, it can be difficult to eliminate them completely. A small amount of residual porosity is often present in the final part, which can influence its mechanical properties like strength and ductility.
Dimensional Shrinkage
The process of densification inherently causes the part to shrink. This shrinkage is predictable but must be precisely calculated and accounted for in the initial design of the mold or green part to achieve the correct final dimensions.
Material and Shape Complexity
Sintering is ideal for creating complex, near-net-shape parts, which minimizes the need for secondary machining. However, the flow of powder during the compaction stage can limit certain geometries, such as those with undercuts or internal threads, without secondary operations.
Making the Right Choice for Your Goal
Deciding to use sintering depends entirely on your material, geometry, and performance requirements.
- If your primary focus is high-melting-point materials: Sintering is often the most practical or only viable method for processing ceramics, refractory metals (like tungsten), and cermets that are too difficult to melt.
- If your primary focus is producing complex shapes at scale: Sintering excels at creating intricate parts with high precision and minimal material waste, making it highly cost-effective for mass production.
- If your primary focus is creating unique material composites: Sintering provides a unique pathway to combine materials that cannot be alloyed through melting, such as metals and ceramics, to create components with specialized properties.
Ultimately, sintering offers a precise method for engineering a material's internal structure from the ground up, enabling the creation of high-performance parts that would be impossible to make otherwise.
Summary Table:
| Sintering Stage | Key Action | Purpose |
|---|---|---|
| 1. Green Part Formation | Powder is mixed with a binder and compacted into shape. | Create the initial fragile form of the final part. |
| 2. Binder Burn-off (Debinding) | Low-temperature heating to remove the temporary binder. | Prepare the powder compact for high-temperature fusion without cracking. |
| 3. High-Temperature Fusion | Heating near (but below) the melting point to enable atomic diffusion. | Fuse powder particles together via necking, increasing density and strength. |
| 4. Controlled Cooling | Gradual cooling of the sintered part. | Solidify the new bonds and set the final microstructure and properties. |
Ready to harness the power of sintering in your lab?
Sintering is essential for creating high-performance parts from metals, ceramics, and unique composites. Achieving consistent, reliable results requires precise temperature control and uniform heating—exactly what KINTEK's advanced laboratory furnaces are designed to deliver.
Whether you are developing new materials or scaling up production of complex components, the right equipment is critical. KINTEK specializes in durable, high-performance lab furnaces and consumables that meet the rigorous demands of sintering processes.
Let's discuss your specific application. Contact our sintering experts today to find the perfect thermal solution for your research or production goals.
Visual Guide

Related Products
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What critical processing conditions are provided by a vacuum hot press sintering furnace? Achieve 98%+ Density.
- What are the advantages of using a vacuum hot pressing sintering furnace? Superior Density for Nanocrystalline Fe3Al
- What are the advantages of using a vacuum hot pressing furnace? Achieve 98.9% Density in Al2O3-TiC Laminated Ceramics
- What is the function of a VHPS system in CoCrFeNiMn alloys? Achieve Near-Theoretical Density and High Purity
- What is the impact factor of powder metallurgy progress? A 2022 Analysis & Context



















