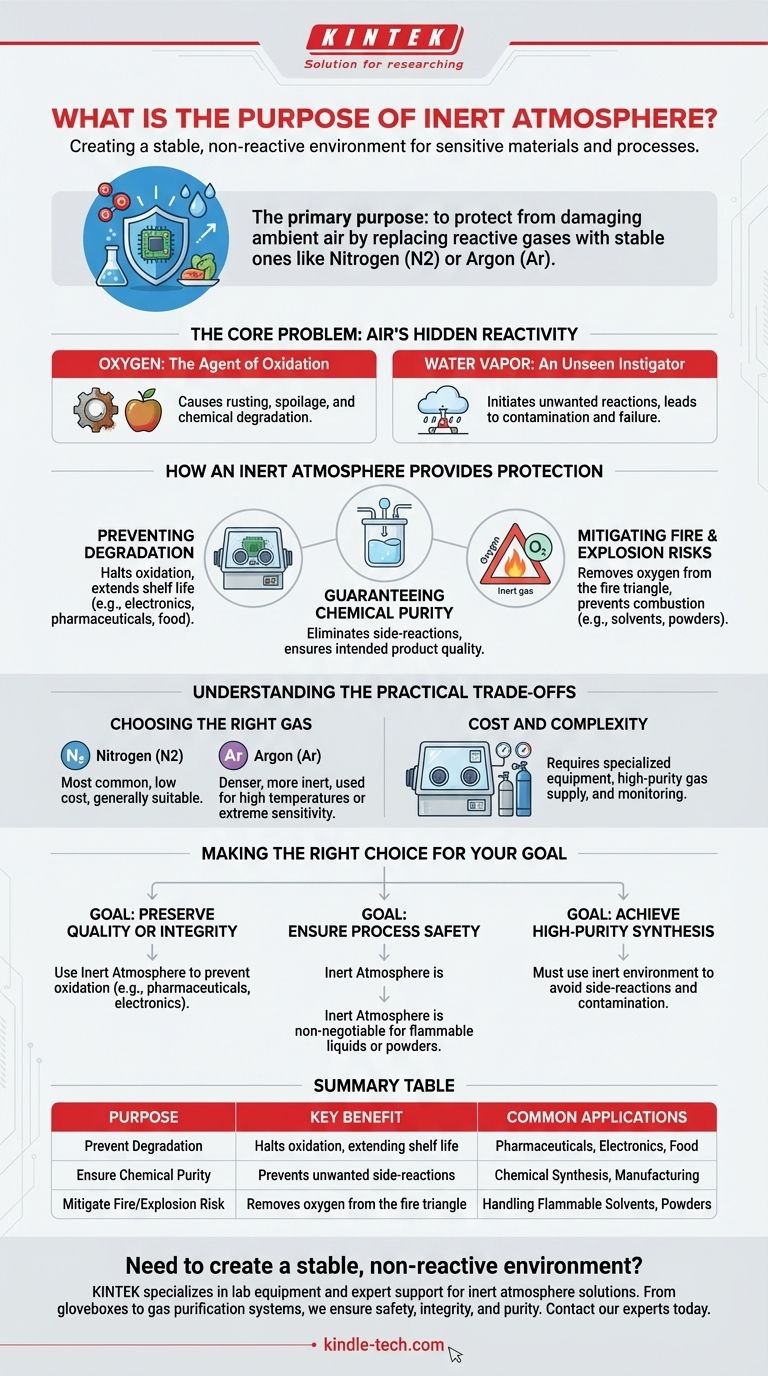
The primary purpose of an inert atmosphere is to create a stable, non-reactive environment that protects sensitive materials or processes from the damaging effects of ambient air. By systematically replacing reactive gases like oxygen and water vapor with a stable gas—most commonly nitrogen or argon—you can prevent unwanted chemical reactions, degradation, and critical safety hazards.
The air we breathe is surprisingly reactive and can easily damage sensitive materials, compromise chemical reactions, or create fire hazards. An inert atmosphere acts as a protective shield, replacing this reactive air with a stable gas to ensure the integrity, safety, and desired outcome of your work.

The Core Problem: Air's Hidden Reactivity
To understand the need for an inert atmosphere, we must first recognize that standard "air" is an active chemical mixture. Two components are the primary sources of interference.
Oxygen: The Agent of Oxidation
Oxygen is highly reactive and seeks to bond with many materials in a process called oxidation. This is the same chemical reaction that causes iron to rust, food to spoil, and the properties of many chemicals to degrade over time.
Water Vapor: An Unseen Instigator
Moisture is pervasive in the atmosphere and can initiate or accelerate a wide range of unwanted chemical reactions. For many advanced materials and chemical reagents, even trace amounts of water can be destructive, leading to contamination or complete process failure.
How an Inert Atmosphere Provides Protection
Introducing an inert gas into a sealed system—such as a glovebox, reaction vessel, or packaging—displaces the reactive air. This fundamentally changes the environment and provides several key benefits.
Preventing Degradation and Ensuring Stability
By removing oxygen, an inert atmosphere directly halts oxidation. This is critical for preserving the quality and extending the shelf life of oxygen-sensitive products, from advanced electronics and pharmaceuticals to specialty foods. It ensures a material's properties remain stable.
Guaranteeing Chemical Purity
In chemical synthesis or manufacturing, reactive atmospheric gases can cause unintended side-reactions. This leads to impurities in the final product, reducing yield and compromising quality. An inert atmosphere ensures that the only reactions occurring are the ones you intend.
Mitigating Fire and Explosion Risks
Fire requires three components: fuel, heat, and an oxidizer (typically oxygen). By replacing oxygen with an inert gas, you remove a key leg of this "fire triangle." This technique, known as inerting, is a critical safety measure when handling flammable solvents, pyrophoric materials, or fine, combustible powders.
Understanding the Practical Trade-offs
While powerful, implementing an inert atmosphere is a deliberate engineering choice with specific considerations. It is not a universal solution but a targeted tool.
Choosing the Right Gas
Nitrogen (N2) is the most common inerting gas due to its low cost and wide availability. Argon (Ar) is denser than nitrogen and even more inert, making it the preferred choice for high-temperature applications (like welding) or with extremely sensitive materials where nitrogen might still react (e.g., with lithium metal).
Cost and Complexity
Maintaining an inert environment requires specialized equipment, such as gloveboxes or Schlenk lines, a reliable supply of high-purity gas, and sensors to monitor oxygen levels. This adds upfront cost and operational complexity compared to working in open air.
The Importance of the Purge
The effectiveness of an inert atmosphere is only as good as the purge. The process must effectively remove virtually all of the original atmosphere. Even trace amounts of oxygen or moisture (measured in parts-per-million) can be sufficient to cause problems in highly sensitive applications.
Making the Right Choice for Your Goal
Deciding to use an inert atmosphere depends entirely on the sensitivity of your materials and the non-negotiable goals of your process.
- If your primary focus is preserving product quality or sample integrity: Use an inert atmosphere to prevent oxidation, which is the leading cause of degradation for pharmaceuticals, food products, and electronic components.
- If your primary focus is ensuring process safety: An inert atmosphere is non-negotiable when handling flammable liquids or fine combustible powders, as it removes the oxygen required for a fire or explosion.
- If your primary focus is achieving high-purity chemical synthesis: You must use an inert environment to prevent unwanted side-reactions with oxygen or water, ensuring your final product is not contaminated.
Ultimately, employing an inert atmosphere is a powerful control used to guarantee stability, safety, and purity in any process where ambient air is a liability.
Summary Table:
| Purpose | Key Benefit | Common Applications |
|---|---|---|
| Prevent Degradation | Halts oxidation, extending shelf life | Pharmaceuticals, Electronics, Food |
| Ensure Chemical Purity | Prevents unwanted side-reactions | Chemical Synthesis, Manufacturing |
| Mitigate Fire/Explosion Risk | Removes oxygen from the fire triangle | Handling Flammable Solvents, Powders |
Need to create a stable, non-reactive environment for your sensitive materials? KINTEK specializes in providing the lab equipment and expert support needed to implement effective inert atmosphere solutions. From gloveboxes to gas purification systems, we help you ensure process safety, product integrity, and chemical purity. Contact our experts today to discuss your specific laboratory requirements and find the right solution for your needs.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the benefits of steam processing on sintered iron? Enhance Strength, Wear, and Corrosion Resistance Today
- What is the main function of an inert atmosphere? Protecting Materials from Oxidation and Degradation
- What is the primary function of an endothermic atmosphere in the heat treatment of steel? Optimize Surface Hardening
- What is the function of inert? Prevent Unwanted Chemical Reactions for a Controlled Process
- What is the role of a high-vacuum or atmosphere protection furnace in treating 304 stainless steel?
- How is a High-Temperature Atmosphere Furnace utilized for zirconium alloy treatment? Enhance Surface Performance
- Which gas is used in annealing furnace? Hydrogen's Role in Protecting Your Materials
- What is the controlled atmosphere brazing process? Achieve High-Volume, High-Strength Metal Joining



















