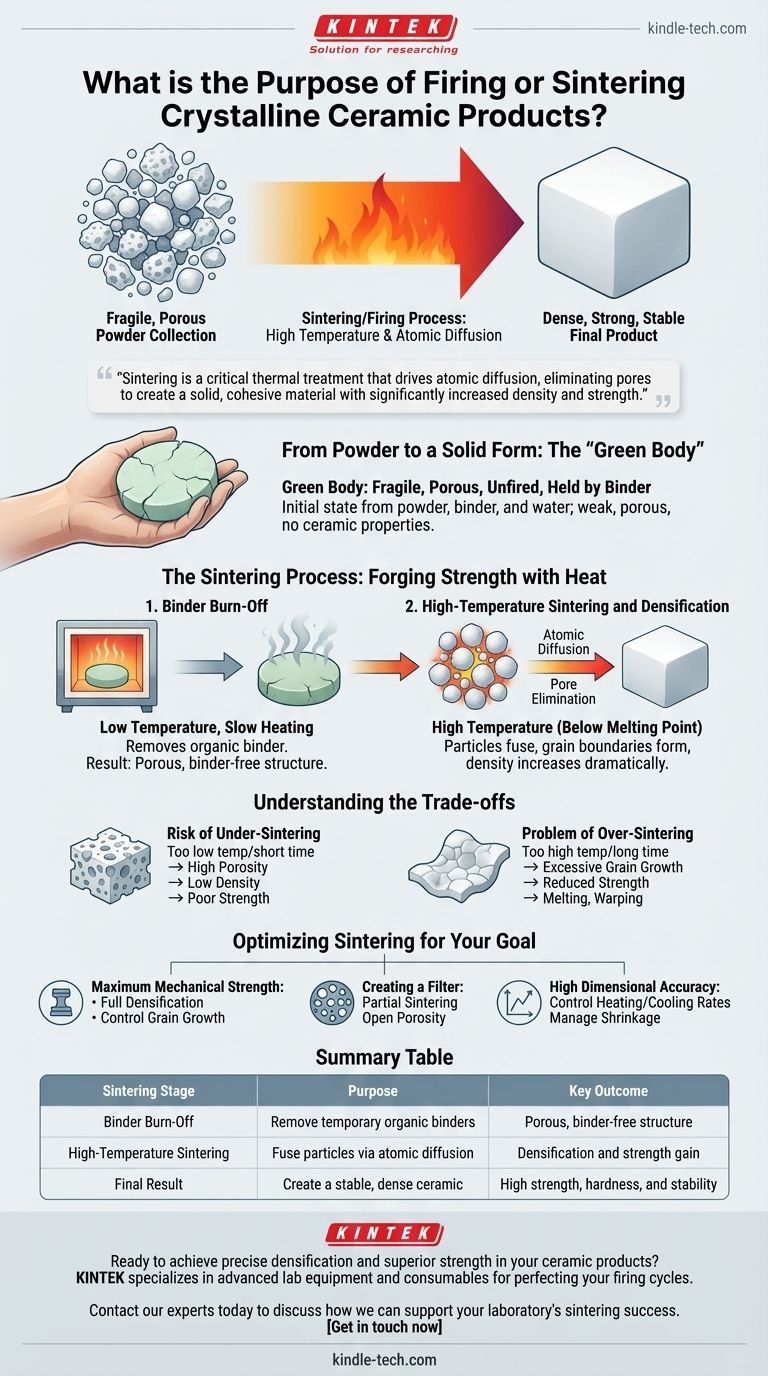
In short, the purpose of firing, or sintering, is to transform a fragile, porous collection of ceramic particles into a dense, strong, and stable final product. This high-temperature process fuses the individual particles together, fundamentally creating the desired mechanical and physical properties of the finished ceramic.
Sintering is not merely a drying or hardening step; it is a critical thermal treatment that drives atomic diffusion between particles. This process eliminates the empty spaces (pores) between them, creating a solid, cohesive material with significantly increased density and strength.

From Powder to a Solid Form: The "Green Body"
To understand why sintering is essential, you must first understand the state of the ceramic material right before it enters the kiln.
What is a "Green Body"?
A ceramic product begins as a carefully mixed powder. This powder is often combined with water and a temporary binder to form a slurry, which is then dried and pressed into the desired shape. This initial, unfired object is known as a "green body."
The Fragility of the Initial State
The green body is extremely fragile. Its particles are only held together mechanically and by the weak adhesive forces of the binder. It is highly porous and possesses none of the strength, hardness, or durability we associate with ceramics.
The Sintering Process: Forging Strength with Heat
The firing process occurs in distinct stages, each with a specific purpose in transforming the weak green body into a robust final part.
Step 1: Binder Burn-Off
The first phase of heating occurs at a relatively low temperature. The primary goal here is to carefully burn off the organic binder that was used to hold the green body together. This must be done slowly to avoid cracking the part as the binder gases escape. After this stage, the object consists of only ceramic particles, but it is still porous and weak.
Step 2: High-Temperature Sintering and Densification
This is the core of the sintering process. The temperature is raised to a point just below the ceramic's melting point. At this high temperature, the atoms at the contact points between individual ceramic particles become highly mobile.
This atomic diffusion causes the particles to fuse together, forming strong bonds and creating continuous "grain boundaries." As the particles merge, the pores between them shrink and are eventually eliminated, dramatically increasing the material's density.
The Result: A Strong, Dense Ceramic
The end result of successful sintering is a process called densification. The elimination of porosity and the formation of a tightly bonded crystalline structure are directly responsible for the final product's key properties: high mechanical strength, hardness, and chemical stability.
Understanding the Trade-offs
Optimizing the sintering process is critical, as deviations in time or temperature can compromise the final product.
The Risk of Under-Sintering
If the temperature is too low or the time is too short, atomic diffusion will be insufficient. The resulting ceramic will retain high levels of porosity, leading to low density, poor mechanical strength, and unacceptable performance.
The Problem of Over-Sintering
Conversely, if the temperature is too high or held for too long, a phenomenon called grain growth can occur. While the part may be dense, excessively large grains can sometimes reduce the material's strength and fracture toughness. In extreme cases, the part may begin to melt, warp, or lose its dimensional accuracy.
Optimizing Sintering for Your Goal
The ideal sintering parameters depend entirely on the intended application of the ceramic component.
- If your primary focus is maximum mechanical strength: The goal is to achieve near-full densification while carefully controlling grain growth through precise temperature and time management.
- If your primary focus is creating a filter: The goal is partial sintering, where you intentionally leave a network of open porosity while still creating strong bonds between particles for structural integrity.
- If your primary focus is high dimensional accuracy: The key is to precisely control the heating and cooling rates to manage the predictable shrinkage that occurs during densification.
Mastering the sintering process is the key to engineering a ceramic's final properties to meet its specific operational demands.
Summary Table:
| Sintering Stage | Purpose | Key Outcome |
|---|---|---|
| Binder Burn-Off | Remove temporary organic binders | Porous, binder-free structure |
| High-Temperature Sintering | Fuse particles via atomic diffusion | Densification and strength gain |
| Final Result | Create a stable, dense ceramic | High strength, hardness, and stability |
Ready to achieve precise densification and superior strength in your ceramic products?
The sintering process is critical to your final product's performance. KINTEK specializes in the advanced lab equipment and consumables needed to perfect your firing cycles, ensuring optimal density, strength, and dimensional accuracy for your specific application—whether for maximum mechanical strength, filtration, or high precision.
Contact our experts today to discuss how we can support your laboratory's sintering success. Get in touch now
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How are samples typically prepared and measured using the diffuse reflection method? Optimize Your Lab's IR Spectroscopy
- How does the calcination process work? Master Thermal Decomposition for Material Purification
- What happens after calcination? A Guide to Material Transformation and Next Steps
- Can calcination be done in a muffle furnace? Yes, for precise air-atmosphere heating.
- What are furnaces usually made of? A Guide to Materials for Extreme Temperatures



















