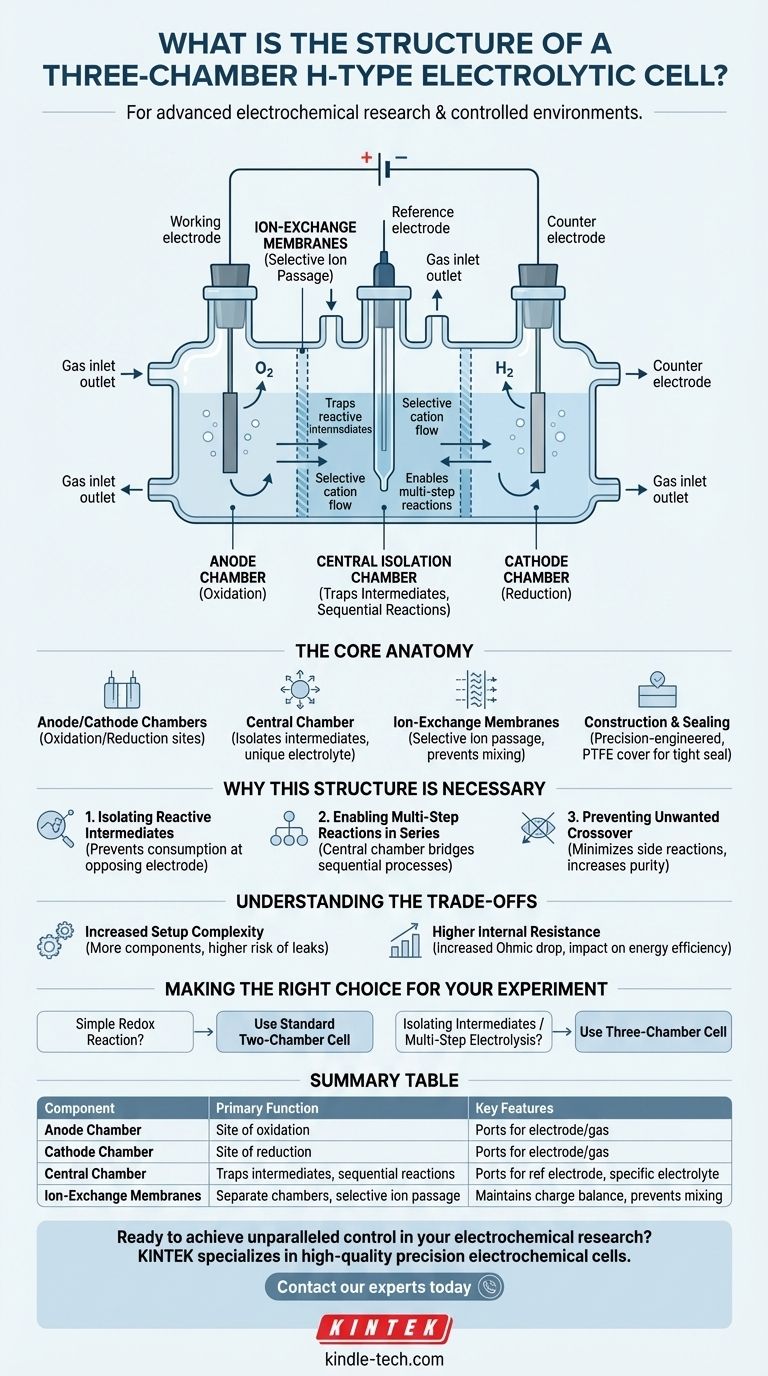
At its core, a three-chamber H-type electrolytic cell is a specialized piece of glassware composed of three distinct, vertically oriented chambers. These chambers—an anode compartment, a cathode compartment, and a central isolation chamber—are separated from each other by ion-exchange membranes, allowing for independent yet interconnected electrochemical environments.
The purpose of the three-chamber design is not simply to add space, but to create a highly controlled system. It enables the isolation, generation, and transformation of chemical species in ways that are impossible in standard two-chamber cells, making it essential for studying complex, multi-step reactions.

The Core Anatomy of the Cell
To understand the cell's function, we must first examine its physical components. Each part serves a specific purpose in controlling the electrochemical process.
The Anode and Cathode Chambers
The two outer chambers function as the standard anode and cathode compartments found in any H-type cell. The anode is where oxidation occurs, and the cathode is where reduction occurs.
These chambers are typically equipped with ports for electrodes and for purging or sampling gases. For example, a common configuration provides one 6.2mm port for the working or counter electrode and two 3.2mm ports for gas inlet/outlet tubes.
The Central Isolation Chamber
This middle chamber is the defining feature of the three-chamber design. It sits between the anode and cathode compartments, physically separating them.
This chamber also includes its own set of ports, often one for an additional electrode (like a reference electrode) and ports for gas. Its primary role is to house a specific electrolyte or to trap reactive intermediates generated at one electrode before they can migrate to the other.
The Role of Ion-Exchange Membranes
The chambers are separated by crucial components: ion-exchange membranes (or sometimes glass frits). These are not impermeable walls.
These membranes are selectively permeable, allowing specific ions (either cations or anions) to pass through while blocking others. This maintains charge neutrality across the cell while preventing the wholesale mixing of the solutions (anolyte and catholyte).
Construction and Sealing
To ensure a controlled atmosphere and prevent leaks, these cells are often precision-engineered. Many designs use a flange-style glass body with a Polytetrafluoroethylene (PTFE) cover. This setup allows for a tight seal, which is critical for experiments sensitive to air or for containing small, precise volumes of solution.
Why This Structure is Necessary
The complexity of the three-chamber design is directly related to the advanced experiments it enables. It provides a level of control that simpler cells cannot match.
Isolating Reactive Intermediates
Many electrochemical reactions produce unstable intermediates. In a two-chamber cell, these species might immediately travel to the opposite electrode and react further, making them difficult to study.
The central chamber can be used to "trap" these intermediates, allowing for their analysis or for them to participate in a subsequent, desired reaction.
Enabling Multi-Step Reactions in Series
The design is ideal for sequential electrolysis. A product generated at the anode can migrate into the central chamber, where it becomes the reactant for a different process, before a final product migrates to the cathode for a third reaction.
This allows researchers to build complex synthesis pathways within a single, integrated electrochemical system.
Preventing Unwanted Crossover
The physical separation provided by the central chamber and two membranes is the most effective way to prevent the reactants and products from the anode and cathode chambers from mixing. This minimizes side reactions and increases the purity and yield of the desired product.
Understanding the Trade-offs
While powerful, the three-chamber design is not always the best choice. Its advantages come with inherent complexities.
Increased Setup Complexity
Managing three separate electrolytes, two membranes, and multiple electrodes requires a more meticulous experimental setup. The risk of leaks or improper assembly is inherently higher than with a simpler two-chamber cell.
Higher Internal Resistance
Every component added to an electrochemical cell increases its internal resistance (Ohmic drop). The second membrane and third volume of electrolyte in this design mean a higher voltage will be required to drive the same amount of current compared to a two-chamber cell, which can impact energy efficiency.
Making the Right Choice for Your Experiment
Selecting the correct cell is critical for experimental success. The choice should be dictated entirely by the complexity of the electrochemical system you intend to study.
- If your primary focus is a simple redox reaction: A standard two-chamber H-cell is often sufficient, more affordable, and easier to operate.
- If your primary focus is to isolate and study reactive intermediates: The three-chamber design is essential for preventing their immediate consumption at the opposing electrode.
- If your primary focus is a sequential, multi-step electrolysis: The central chamber provides the ideal, controlled environment to bridge two distinct electrochemical processes.
Ultimately, the three-chamber H-type cell is a sophisticated tool that gives the researcher precise control over the reaction environment.
Summary Table:
| Component | Primary Function | Key Features |
|---|---|---|
| Anode Chamber | Site of oxidation reaction | Ports for electrode and gas inlet/outlet |
| Cathode Chamber | Site of reduction reaction | Ports for electrode and gas inlet/outlet |
| Central Isolation Chamber | Traps intermediates; enables sequential reactions | Ports for reference electrode/gas; houses specific electrolyte |
| Ion-Exchange Membranes | Separate chambers; allow selective ion passage | Maintains charge balance; prevents solution mixing |
Ready to achieve unparalleled control in your electrochemical research? The sophisticated design of a three-chamber H-cell is essential for studying reactive intermediates and multi-step reactions. KINTEK specializes in high-quality laboratory equipment, including precision electrochemical cells, to meet your exact research needs. Contact our experts today to discuss how our solutions can enhance your lab's capabilities and drive your discoveries forward.
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide
- What preparation steps are needed before starting an experiment with an H-type electrolytic cell? A Guide to Safe and Accurate Results
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What should be observed during an experiment with the H-type electrolytic cell? Key Monitoring for Precise Results
- What are the proper storage conditions for an H-type electrolytic cell? Ensure Long-Term Reliability and Accurate Results



















