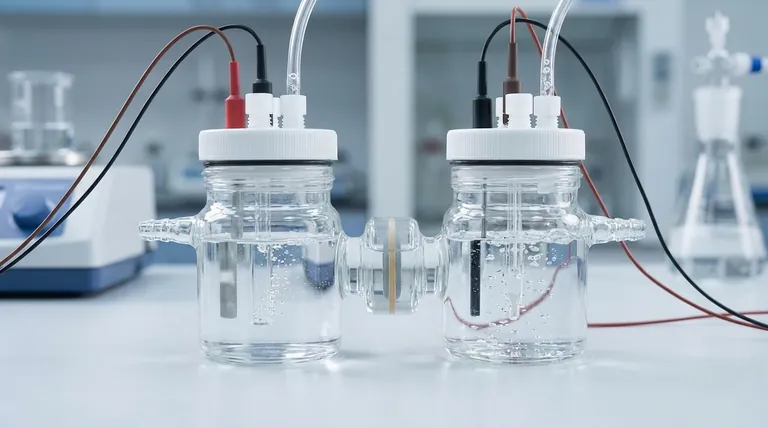
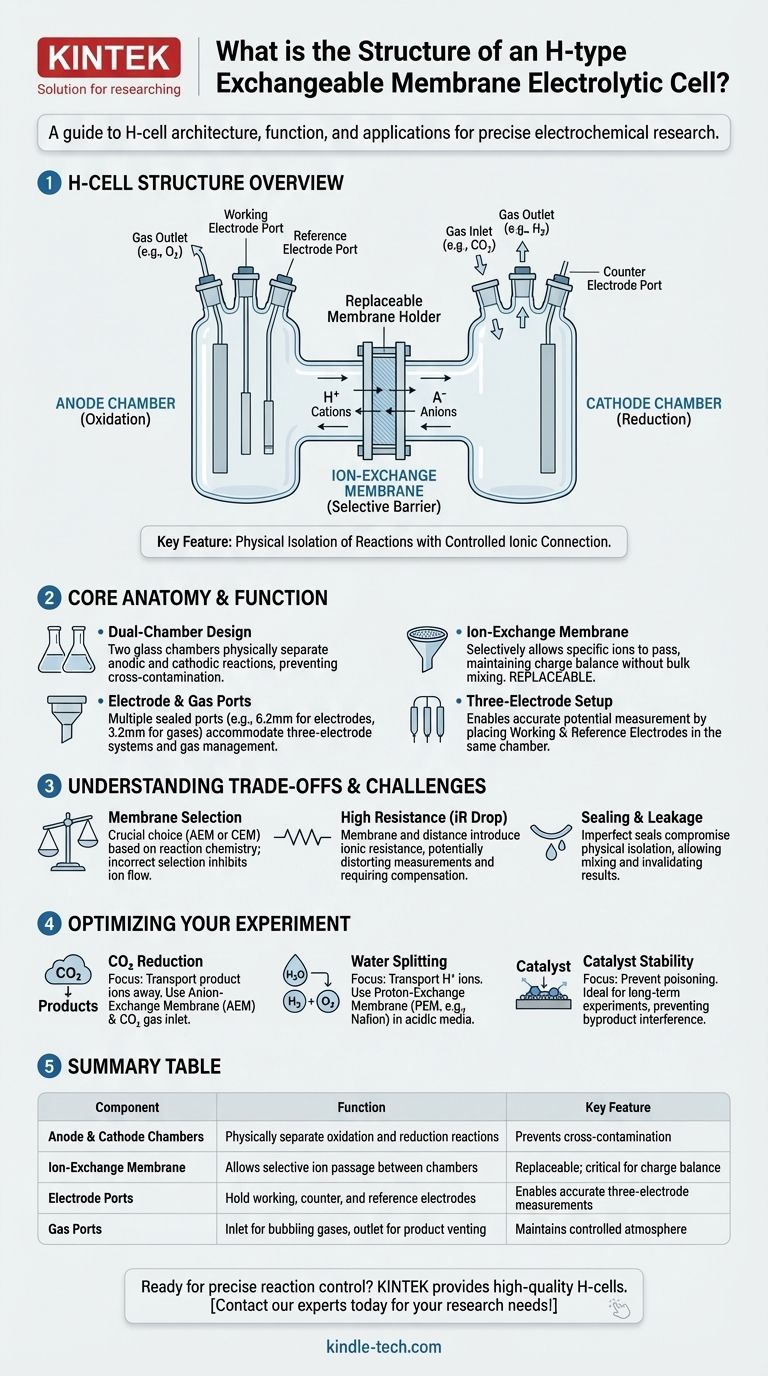
An H-type exchangeable membrane electrolytic cell is a specialized piece of electrochemical apparatus composed of two distinct chambers—an anode chamber and a cathode chamber—physically separated by a replaceable ion-exchange membrane. This design is engineered to house a complete three-electrode system (working, counter, and reference electrodes) and includes ports for gas inlet and outlet, enabling precise control over electrochemical reactions.
The core purpose of the H-cell's structure is to physically isolate the reactions at the anode and cathode while maintaining a controlled ionic connection through the membrane. This separation prevents cross-contamination of reactants and products, which is critical for obtaining accurate and reproducible experimental data.
The Core Anatomy of the H-Cell
The name "H-cell" comes from its characteristic shape, which resembles the letter H. This design is not arbitrary; it is a functional architecture where each component serves a specific purpose.
The Dual-Chamber Design
The cell is fundamentally two separate glass chambers joined by a central bridge. One chamber is designated for the anodic reaction (oxidation), and the other is for the cathodic reaction (reduction). This clear physical separation is the cell's primary feature.
The Ion-Exchange Membrane: The Critical Separator
Positioned in the bridge connecting the two chambers is a holder for an ion-exchange membrane. This membrane is the heart of the cell's function.
Its role is to act as a selective barrier, allowing only specific types of ions (either positive cations or negative anions) to pass between the chambers. This prevents the bulk mixing of the electrolytes, reactants, and products from either side. The membrane is also replaceable, allowing researchers to choose one that suits the specific ions involved in their experiment.
Electrode and Gas Ports
Each chamber is sealed and features several ports to accommodate the necessary hardware. A typical configuration includes:
- Electrode Ports: Usually 6.2mm in diameter, these are designed to hold the working, counter, and reference electrodes.
- Gas Ports: Smaller ports, often 3.2mm, are used to bubble gases into the electrolyte (e.g., delivering CO₂ for reduction) or to vent gaseous products (e.g., H₂ or O₂).
The standard arrangement places the working electrode and reference electrode in one chamber, while the counter electrode resides in the other.
How the Structure Enables Precise Experiments
The H-cell's anatomy directly translates into higher-quality electrochemical measurements by solving several common experimental challenges.
Isolating Anodic and Cathodic Reactions
The most significant advantage is the prevention of crossover. For example, in water splitting, oxygen evolved at the anode is prevented from reaching the cathode, where it could interfere with hydrogen evolution. This ensures the products and catalysts on each side remain pure and unaffected by side reactions.
Maintaining Charge Neutrality
As a reaction proceeds, ions are consumed or produced at each electrode, creating a charge imbalance. The ion-exchange membrane allows counter-ions to flow from one chamber to the other, balancing the charge and completing the electrical circuit. Without this ionic conductivity, the reaction would quickly stop.
Supporting a Three-Electrode Setup
The separate ports allow for a proper three-electrode configuration. Placing the reference electrode in the same chamber as the working electrode is crucial for accurately measuring the potential of the working electrode without interference from voltage drops occurring across the membrane.
Understanding the Trade-offs and Pitfalls
While powerful, the H-cell's design comes with considerations that every researcher must manage.
Membrane Selection is Critical
The choice between an anion-exchange membrane (AEM) and a cation-exchange membrane (CEM) is dictated by the reaction chemistry. Using the wrong membrane will inhibit ion flow, stop the reaction, and render the results invalid.
Potential for High Resistance
The membrane itself, along with the physical distance between the anode and cathode, introduces significant ionic resistance (known as iR drop). This resistance can distort electrochemical measurements and increase the energy required to drive the reaction. It is a known factor that must often be compensated for in data analysis.
Sealing and Leakage
A perfect seal around the exchangeable membrane is vital. Any leakage between the two chambers defeats the primary purpose of the cell, allowing electrolytes and products to mix and compromising the integrity of the experiment.
Making the Right Choice for Your Experiment
The H-cell is a versatile tool, but its configuration must be matched to your specific research goal.
- If your primary focus is CO₂ reduction: You will need an anion-exchange membrane to transport product ions (like formate or carbonate) away from the cathode and gas ports to deliver CO₂.
- If your primary focus is water splitting: You will typically use a proton-exchange membrane (like Nafion) to transport H⁺ ions from the anode to the cathode in acidic media.
- If your primary focus is catalyst stability testing: The H-cell's separation is ideal, as it prevents byproducts from one electrode from dissolving and poisoning the catalyst on the other electrode over long-term experiments.
Ultimately, the H-type cell provides an essential framework for controlling and understanding complex electrochemical systems with precision.
Summary Table:
| Component | Function | Key Feature |
|---|---|---|
| Anode & Cathode Chambers | Physically separate oxidation and reduction reactions | Prevents cross-contamination of reactants/products |
| Ion-Exchange Membrane | Allows selective ion passage between chambers | Replaceable; critical for charge balance |
| Electrode Ports (6.2mm) | Hold working, counter, and reference electrodes | Enables accurate three-electrode measurements |
| Gas Ports (3.2mm) | Inlet for bubbling gases (e.g., CO₂), outlet for product venting | Maintains controlled atmosphere |
Ready to achieve precise reaction control in your lab? The H-type exchangeable membrane electrolytic cell is essential for researchers focusing on CO₂ reduction, water splitting, and catalyst stability testing. At KINTEK, we specialize in high-quality lab equipment and consumables tailored to your electrochemical needs. Our expertise ensures you get the right configuration for accurate, reproducible results. Contact our experts today to discuss how our H-cells can enhance your research!
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- H Type Electrolytic Cell Triple Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
People Also Ask
- What is a double-layer water-bath electrolytic cell? Achieve Precise Temperature Control for Your Electrolysis
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide
- What is a H type cell? A Guide to Divided Electrochemical Cells for Accurate Experiments
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control



















