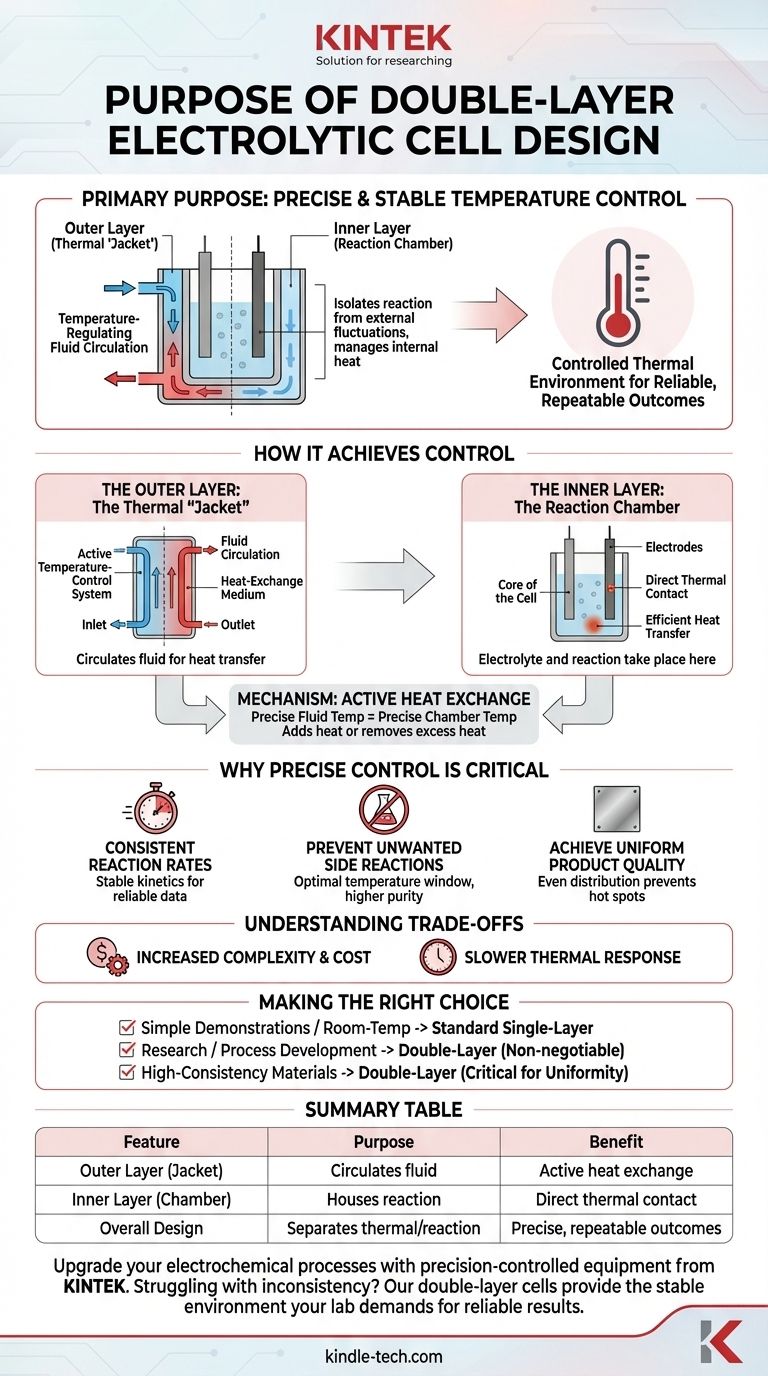
The primary purpose of a double-layer design in an electrolytic cell is to provide precise and stable temperature control for the reaction. The outer layer acts as a jacket through which a temperature-regulating fluid circulates, while the inner layer contains the electrolyte, effectively isolating the reaction from external temperature fluctuations and managing any heat generated internally.
Electrolytic reactions are highly sensitive to temperature. The double-layer design solves this by creating a controlled thermal environment, transforming the cell from a simple container into a precision instrument for ensuring reliable and repeatable experimental outcomes.

How the Double-Layer Design Achieves Control
The structure functions as a "cell within a cell," where each layer has a distinct role. This separation of functions is the key to its effectiveness.
The Outer Layer: The Thermal "Jacket"
The outer layer is not merely for insulation; it is an active temperature-control system.
It features an inlet and an outlet, allowing a fluid, typically water from an external circulating bath, to flow continuously through the space between the two layers. This circulating fluid acts as a heat-exchange medium.
The Inner Layer: The Reaction Chamber
This is the core of the cell, where the electrolyte and electrodes are housed and the electrochemical reaction takes place.
The walls of this inner chamber are in direct thermal contact with the fluid circulating in the outer jacket, allowing for efficient heat transfer.
The Mechanism: Active Heat Exchange
By precisely controlling the temperature of the circulating fluid, you can dictate the temperature of the inner reaction chamber.
The system can either add heat to the electrolyte for reactions that need to be run at elevated temperatures or, more commonly, remove excess heat generated by the electrolytic process itself.
Why Precise Temperature Control is Critical
Controlling temperature isn't just a minor improvement; it's fundamental to achieving accurate and high-quality results in many electrochemical applications.
To Ensure Consistent Reaction Rates
Electrochemical reaction kinetics are strongly dependent on temperature. Unstable temperatures lead to fluctuating reaction speeds, making it impossible to obtain reliable data or consistent product yields.
To Prevent Unwanted Side Reactions
Many processes have an optimal temperature window. If the cell overheats due to the reaction's own energy output (Joule heating), it can trigger undesirable side reactions, reducing the purity and yield of the desired product.
To Achieve Uniform Product Quality
The jacket ensures an even temperature distribution across the entire electrode surface. This prevents localized "hot spots" that can cause uneven metal deposition, inconsistent coatings, or flawed organic synthesis.
Understanding the Trade-offs
While highly effective, the double-layer design is not always the default choice. Its benefits come with practical considerations.
Increased Complexity and Cost
A double-layer, or jacketed, cell is inherently more complex and expensive than a standard single-wall beaker. It also requires an external heated/chilled circulating bath, which adds to the overall cost and footprint of the setup.
Slower Thermal Response
Because heat must be transferred through the inner glass wall and into the bulk electrolyte, the system's response to a change in the set temperature is not instantaneous. This is a minor consideration but relevant for experiments requiring rapid temperature cycling.
Making the Right Choice for Your Goal
Selecting the appropriate cell depends entirely on the requirements of your experiment or process.
- If your primary focus is simple demonstrations or room-temperature reactions: A standard, single-layer cell is often sufficient, simpler, and more cost-effective.
- If your primary focus is research, process development, or temperature-sensitive synthesis: The precise control of a double-layer cell is non-negotiable for obtaining accurate and repeatable results.
- If your primary focus is producing high-consistency materials (e.g., electroplating): The uniform temperature distribution provided by a jacketed cell is critical for ensuring consistent product quality.
Ultimately, the double-layer design is the essential tool that enables the transition from qualitative observation to precise, quantitative electrochemical engineering.
Summary Table:
| Feature | Purpose | Benefit |
|---|---|---|
| Outer Layer (Jacket) | Circulates temperature-regulating fluid | Active heat exchange, isolates reaction from external fluctuations |
| Inner Layer (Chamber) | Houses electrolyte and electrodes | Direct thermal contact for efficient temperature control |
| Overall Design | Separates thermal management from reaction space | Enables precise, repeatable experimental outcomes |
Upgrade your electrochemical processes with precision-controlled equipment from KINTEK.
Struggling with inconsistent reaction rates or unwanted side products due to temperature fluctuations? Our double-layer electrolytic cells are engineered to provide the stable thermal environment your research or production demands. By ensuring uniform temperature distribution, KINTEK lab equipment helps you achieve reliable, repeatable results in electroplating, organic synthesis, and material development.
We specialize in serving laboratories that require accuracy and consistency. Let us help you enhance your experimental outcomes. Contact our experts today to find the perfect solution for your specific application!
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- What optical features does the H-type electrolytic cell have? Precision Quartz Windows for Photoelectrochemistry
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What is a H type cell? A Guide to Divided Electrochemical Cells for Accurate Experiments
- What is the structure of an H-type exchangeable membrane electrolytic cell? A Guide to Precise Electrochemical Separation



















