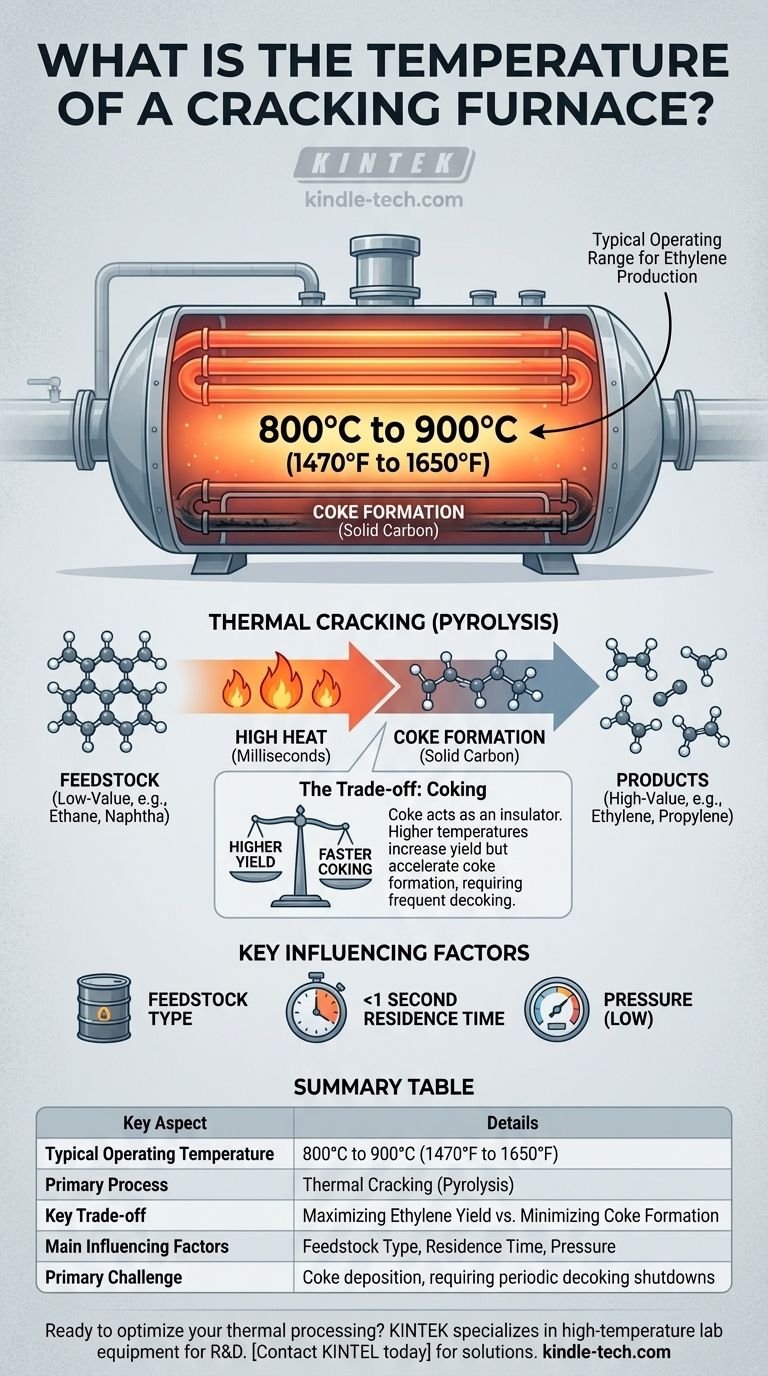
In the petrochemical industry, a typical steam cracking furnace used for producing ethylene operates with a tube outlet temperature in the range of 800°C to 900°C (1470°F to 1650°F). This temperature is not a fixed value but is precisely controlled based on the specific hydrocarbon feedstock and the desired products.
The term "cracking furnace" refers to a unit's process function—thermally breaking down hydrocarbons—not a specific type of heating technology. The critical takeaway is that the temperature is a carefully managed variable designed to optimize the yield of valuable chemicals while minimizing undesirable byproducts like coke.

Understanding the "Cracking" Process
To understand the temperature, you must first understand the goal. A cracking furnace is a chemical reactor whose sole purpose is to transform low-value hydrocarbons into high-value ones.
What is Thermal Cracking?
Thermal cracking, or pyrolysis, uses high heat to break the strong chemical bonds within large hydrocarbon molecules. This process "cracks" them into smaller, more valuable molecules.
For example, ethane (C₂H₆) is cracked to produce ethylene (C₂H₄), a foundational building block for plastics. Naphtha, a heavier feedstock, can be cracked into ethylene, propylene, and other useful chemicals.
Why This Specific Temperature Range?
The 800°C to 900°C range is a chemical sweet spot for light feedstocks.
At these temperatures, the energy is sufficient to break the carbon-carbon (C-C) and carbon-hydrogen (C-H) bonds efficiently. The reaction happens in milliseconds.
If the temperature is too low, the cracking reaction is too slow to be economical. If it's too high, it promotes unwanted side reactions, primarily producing excess methane and coke (solid carbon).
Key Factors Influencing Temperature
The ideal temperature is not a single number. It is a dynamic variable influenced by several factors:
- Feedstock: Heavier feedstocks (like gas oil) require different temperature profiles and longer residence times than lighter ones (like ethane).
- Residence Time: This is the extremely short duration (often less than a second) that the hydrocarbon spends in the hottest part of the furnace. It is precisely controlled along with temperature.
- Pressure: Cracking is typically done at low hydrocarbon partial pressures, which favors the formation of olefins like ethylene.
Differentiating Furnace Types from Processes
The references you provided mention muffle and induction furnaces, which can achieve very high temperatures—up to 1800°C in some cases. However, it is crucial to distinguish the heating method from the industrial process.
The Role of an Industrial Cracking Furnace
A commercial cracking furnace is a massive, direct-fired piece of equipment. It contains coils of metal alloy tubes through which the hydrocarbon feedstock flows.
Burners lining the furnace walls heat these tubes to the exact temperature needed to initiate the cracking reaction inside. The furnace's job is simply to deliver this precise and intense heat profile.
What About Induction or Muffle Furnaces?
Induction and muffle furnaces are defined by their heating technology.
An induction furnace heats conductive material with electromagnetic induction, while a muffle furnace often uses an external flame to heat a separated chamber, providing a controlled atmosphere.
While these furnaces can certainly reach the temperatures required for cracking, they are not the technology used for large-scale industrial ethylene production. They are more common in laboratories, foundries, or specialized material processing applications.
Understanding the Primary Trade-off: Coking
Operating at such high temperatures creates a significant operational challenge that defines the entire process.
The Inevitable Problem of Coke
At cracking temperatures, some hydrocarbon molecules decompose completely into pure carbon, or coke. This solid coke deposits on the inner wall of the furnace tubes.
The Impact of Coking
Coke acts as an insulator. As it builds up, it reduces the transfer of heat from the furnace burners to the hydrocarbons inside the tube.
To compensate, operators must increase the furnace's firing temperature to maintain the required process temperature. This eventually reaches a limit, increases mechanical stress on the tubes, and reduces efficiency.
Balancing Yield vs. Run Length
This creates the core operational trade-off. Running at higher temperatures can increase the yield of valuable products like ethylene. However, higher temperatures also dramatically accelerate the rate of coke formation.
Faster coking means the furnace must be taken offline more frequently for a "decoking" procedure, where the carbon is burned off with steam and air. This results in lost production.
Making the Right Choice for Your Goal
The optimal cracking furnace temperature is not a static number but a strategic decision based on economic and operational goals.
- If your primary focus is maximizing ethylene yield: You will operate at the higher end of the temperature range (e.g., 875°C+) and accept shorter run times between decoking shutdowns.
- If your primary focus is operational stability and long run times: You may operate at a slightly lower temperature to minimize coking rates, extending the production cycle at the cost of a marginally lower yield.
- If you are processing a heavier, more complex feedstock: The entire temperature profile, residence time, and steam dilution ratio must be co-optimized to manage both product yield and severe coking tendencies.
Ultimately, mastering a cracking furnace is about precisely controlling temperature to manage the fundamental chemical trade-off between production and degradation.
Summary Table:
| Key Aspect | Details |
|---|---|
| Typical Operating Temperature | 800°C to 900°C (1470°F to 1650°F) |
| Primary Process | Thermal Cracking (Pyrolysis) |
| Key Trade-off | Maximizing Ethylene Yield vs. Minimizing Coke Formation |
| Main Influencing Factors | Feedstock Type, Residence Time, Pressure |
| Primary Challenge | Coke deposition, requiring periodic decoking shutdowns |
Ready to optimize your thermal processing operations?
Whether you're developing new processes in the lab or scaling up production, precise temperature control is critical. KINTEK specializes in high-temperature lab equipment, including furnaces capable of reaching and maintaining the extreme temperatures required for research and development in petrochemicals, materials science, and more.
Our experts can help you select the right equipment to achieve the precise thermal profiles you need, helping you maximize yield and efficiency while managing operational challenges.
Contact KINTEL today to discuss your specific high-temperature application and how our solutions can drive your success.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the role of a high-temperature tube furnace in the synthesis of Mo2C catalysts? Achieve Precise Carbonization
- What role does a high-temperature tube furnace play in the CVD synthesis of Fe-C@C nanoparticles? Key Insights
- What temperature is needed for pyrolysis waste? A Guide to Optimizing Your Waste-to-Value Process
- What is the necessity of using a vacuum tube furnace for Boron Carbide (B4C)? Achieve Optimal Preform Sintering
- Why are quartz sealing tubes and argon protection required during annealing of Fe-Mn-Cr alloys? Ensure Alloy Integrity
- What role does a tube furnace play in processing LiCoO2 (LCO) cathode thin films? Unlock Peak Battery Performance
- What are furnace tubes made of? Choose the Right Material for Your Lab's Thermal Processes
- Why is a tube reduction furnace required for Fe-Cu powders? Eliminate Oxides for Superior Sintering Results



















