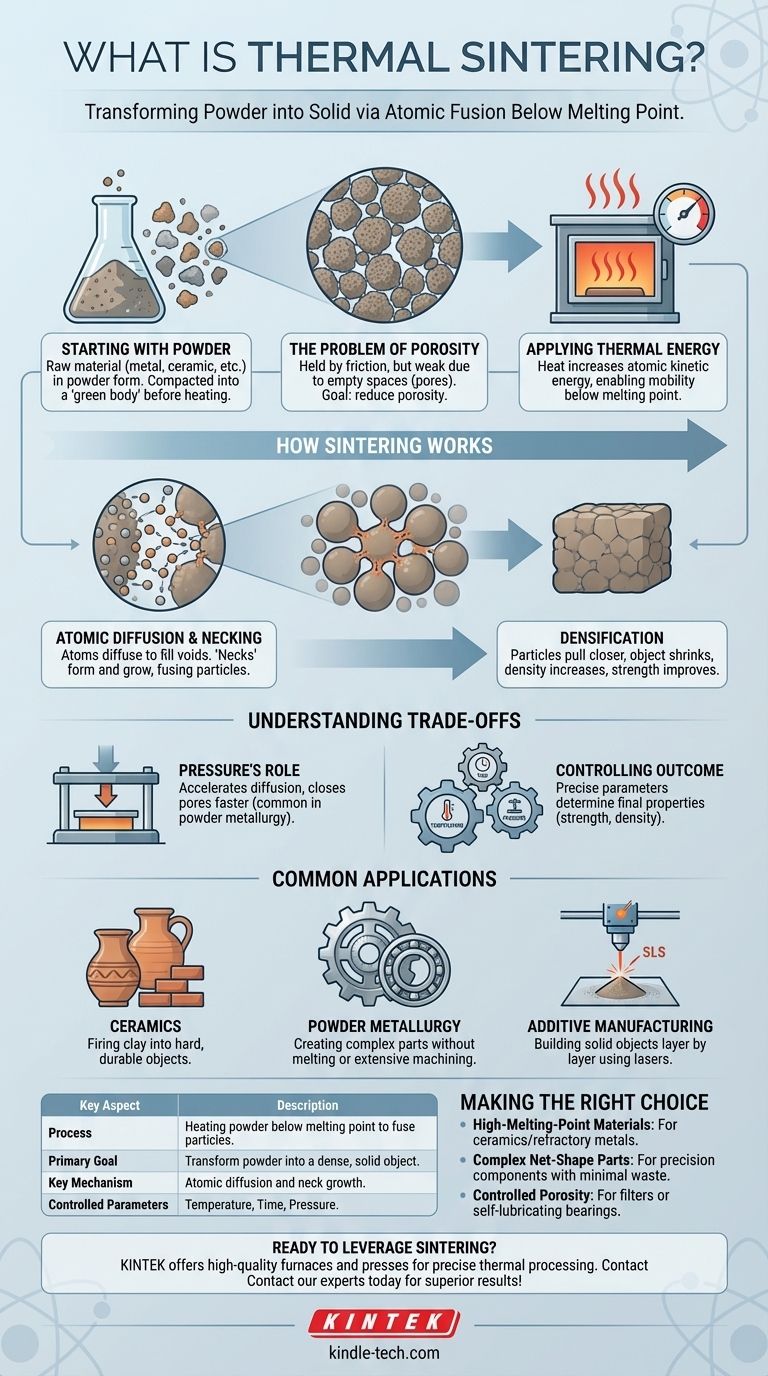
At its core, thermal sintering is a manufacturing process that transforms a collection of powder particles into a solid, dense object. It achieves this by applying high heat, often combined with pressure, at a temperature below the material's actual melting point. Instead of melting the material into a liquid, sintering encourages the individual particles to bond and fuse on an atomic level, creating a strong, coherent mass.
The crucial concept to grasp is that sintering is not melting. It is a solid-state process where heat energizes atoms, allowing them to diffuse across particle boundaries to eliminate the empty spaces between them, resulting in a stronger, denser final product.

The Fundamental Goal: From Powder to Solid
Starting with a Powdered Material
The sintering process always begins with a raw material in powder form. This could be a metal, a ceramic, a plastic, or a composite material. The initial powder is often compacted into a desired shape, known as a "green body," before heating.
The Problem of Porosity
A compacted powder is held together by friction but is mechanically weak because of the vast number of tiny empty spaces, or pores, between the particles. The primary objective of sintering is to significantly reduce or eliminate this porosity.
Applying Thermal Energy
Heat is the main catalyst in the sintering process. This thermal energy increases the kinetic energy of the atoms within the powder particles, making them mobile enough to move and form new bonds.
How Sintering Actually Works
Avoiding the Melting Point
It is critical to understand that the sintering temperature is always kept below the material's melting point. If the material were to melt, the process would be casting, not sintering. This allows for the creation of parts from materials with exceptionally high melting points.
The Mechanism: Atomic Diffusion
The applied heat allows atomic diffusion to occur. Atoms migrate from the bulk of the particles to the points of contact between them. As atoms move to fill the voids, the particles begin to fuse together.
Necking and Densification
The initial points of fusion between particles are called "necks." As the sintering process continues, these necks grow wider, pulling the particles closer together. This causes the entire object to shrink and its density to increase, resulting in a much stronger final component.
Understanding the Trade-offs
The Role of Pressure
While heat is the primary driver, pressure is often applied to accelerate the process. Pressure forces the particles into closer contact, which enhances the rate of atomic diffusion and helps to close up pores more effectively. This combination is common in powder metallurgy.
Controlling the Outcome
The final properties of a sintered part—such as strength, hardness, and density—are directly controlled by three main parameters: temperature, time, and pressure. Fine-tuning these variables allows engineers to precisely tailor the material characteristics for a specific application.
Common Applications
Sintering is fundamental to many industries. It is the process used to fire pottery and ceramics, transforming clay into a hard, durable object. In powder metallurgy, it is used to create complex metal parts like self-lubricating bearings and gears without the need for melting or extensive machining. Modern additive manufacturing (3D printing) processes like Selective Laser Sintering (SLS) use a laser to sinter layers of powder, building a solid object from the ground up.
Making the Right Choice for Your Goal
Understanding the core purpose of sintering helps determine when it is the most effective manufacturing method.
- If your primary focus is working with high-melting-point materials: Sintering is the go-to process for creating solid parts from ceramics or refractory metals that are impractical to melt and cast.
- If your primary focus is creating complex net-shape parts: Powder metallurgy uses sintering to produce intricate components with high precision, minimizing material waste and the need for post-machining.
- If your primary focus is controlling material porosity: Sintering provides exceptional control over the final density, essential for creating components like filters or porous bearings designed to hold lubricant.
By mastering heat and pressure below the melting point, sintering provides a powerful method for turning simple powders into high-performance engineered components.
Summary Table:
| Key Aspect | Description |
|---|---|
| Process | Heating powder below its melting point to fuse particles. |
| Primary Goal | Transform powdered material into a dense, solid object. |
| Key Mechanism | Atomic diffusion and neck growth between particles. |
| Common Applications | Powder metallurgy, ceramics, additive manufacturing (SLS). |
| Controlled Parameters | Temperature, time, and pressure. |
Ready to leverage sintering for your lab's material processing needs?
KINTEK specializes in providing high-quality lab equipment, including furnaces and presses essential for precise thermal sintering processes. Whether you are developing new materials in powder metallurgy, advancing ceramic applications, or innovating with additive manufacturing, our solutions are designed to deliver the controlled heating and pressure required for superior results.
Contact our experts today to discuss how we can support your specific sintering requirements and help you achieve stronger, more complex components with greater efficiency.
Visual Guide
Related Products
- Vacuum Dental Porcelain Sintering Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Spark Plasma Sintering Furnace SPS Furnace
People Also Ask
- What are five applications of soldering? From Electronics to Art, Master Material Joining
- Why are high-precision vacuum sintering furnaces preferred over traditional methods for biofunctional dental ceramics?
- What is the function of a porcelain furnace? Precision Firing for Lifelike Dental Restorations
- What is the advantage of firing porcelain in a vacuum? Achieve Denser, Stronger, and More Aesthetic Dental Restorations
- How does precision temperature control impact TiAl alloy sintering? Master Microstructure Development



















