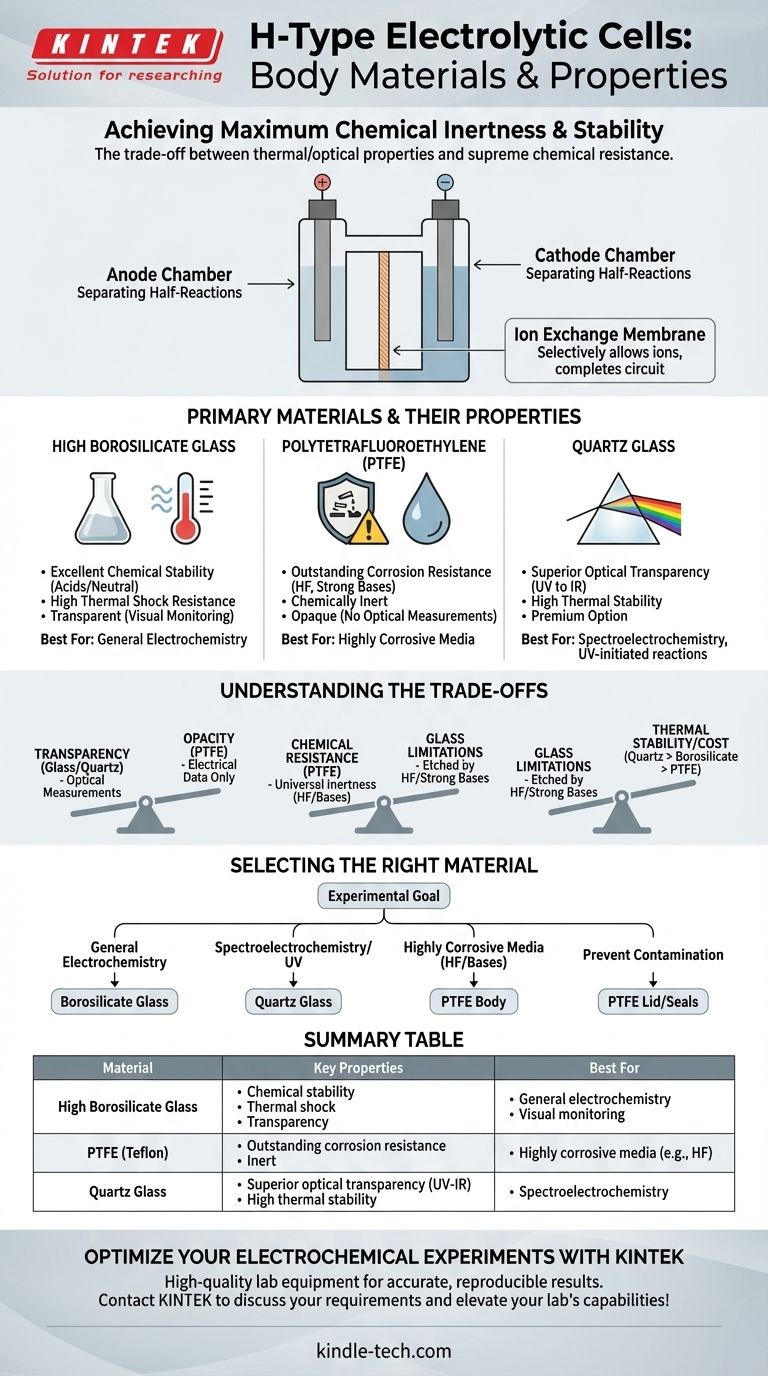
At its core, the body of an H-type electrolytic cell is constructed from materials chosen for maximum chemical inertness and stability under specific experimental conditions. The most common materials are high borosilicate glass and Polytetrafluoroethylene (PTFE), with quartz glass serving as a specialized alternative. The selection is dictated by the need for thermal stability, optical transparency, and resistance to highly corrosive electrolytes.
The material you choose for your H-type cell is not just a container; it is an active variable in your experiment. The decision hinges on a critical trade-off between the thermal and optical properties of glass and the supreme chemical resistance of PTFE.

The Purpose of the H-Type Cell Design
An H-type cell's design is fundamental to its function in many electrochemical experiments. The material of the body must support this unique structure.
Separating Anode and Cathode Chambers
The signature "H" shape divides the cell into two distinct chambers. This separation is crucial for isolating the reactions occurring at the anode from those at the cathode.
By preventing the mixing of reactants or products, you can study each half-reaction independently, which is essential for accurate measurements and mechanistic studies.
The Role of the Ion Exchange Membrane
A replaceable ion exchange membrane is typically placed at the junction between the two chambers. This membrane is the bridge that completes the electrical circuit.
It allows specific ions to pass between the anode and cathode compartments while blocking other species, ensuring the integrity and accuracy of the experiment.
Providing a Stable, Contained Environment
Ultimately, the cell body's primary function is to hold the electrolyte and electrodes securely. It must do so without leaching impurities or reacting with the chemical system, which would compromise the experimental results.
Primary Materials and Their Properties
The choice of material directly impacts the types of experiments you can run. Each has a distinct profile of strengths and weaknesses.
High Borosilicate Glass: The Standard Choice
High borosilicate glass is the most common material for H-type cell bodies due to its excellent balance of properties.
It offers very good chemical stability in the presence of most acids and neutral solutions, coupled with high thermal shock resistance, allowing it to be used in a wide range of temperatures. Its transparency is also a key benefit for visually monitoring the reaction.
Polytetrafluoroethylene (PTFE): The Corrosion Specialist
PTFE, commonly known by the trade name Teflon, is used when chemical resistance is the absolute priority.
It has outstanding corrosion resistance, remaining inert even when exposed to extremely aggressive chemicals like concentrated acids and strong bases. PTFE is often the material of choice for the cell's lid and seals to prevent any contamination.
Quartz Glass: The Optical Specialist
Quartz glass is a premium option reserved for specific applications, primarily spectroelectrochemistry.
Its key advantage is superior optical transparency across the entire light spectrum, from ultraviolet (UV) to infrared (IR). While it also has excellent chemical resistance (except to hydrofluoric acid), its high cost means it is only used when broadband optical access is non-negotiable.
Understanding the Trade-offs
Choosing a material is a matter of prioritizing the most critical parameter for your specific experimental goal.
Transparency vs. Opacity
Glass (both borosilicate and quartz) is transparent, allowing for visual observation of electrode color changes, bubble formation, or precipitation. More importantly, it is essential for experiments that couple spectroscopy with electrochemistry.
PTFE is opaque, making it impossible to perform optical measurements through the cell body. Its use is limited to experiments where only electrical data is being collected.
Chemical Resistance: When Glass Is Not Enough
While borosilicate glass is highly stable, it can be etched by hydrofluoric acid (HF) and strong alkaline (basic) solutions, especially at elevated temperatures.
In these highly corrosive environments, PTFE is the necessary choice. Its near-universal chemical inertness ensures the cell body will not degrade or contaminate the experiment.
Thermal Stability and Cost
High borosilicate glass has an excellent working temperature range. Quartz has even better thermal stability but at a significantly higher cost.
PTFE has a lower maximum operating temperature compared to glass, which can be a limiting factor in some high-temperature electrochemical systems.
Selecting the Right Material for Your Experiment
Your choice should be a direct reflection of your experimental requirements.
- If your primary focus is general electrochemistry: High borosilicate glass offers the best balance of performance, visibility, and cost.
- If your primary focus is spectroelectrochemistry or UV-initiated reactions: Quartz glass is the only material that provides the necessary broad-spectrum optical transparency.
- If your primary focus is working with highly corrosive media (like HF or strong bases): A PTFE body is essential to ensure the integrity of the cell and the purity of your results.
- If your primary focus is preventing contamination from the lid or seals: Ensure all wetted components, especially the lid and electrode fittings, are made from inert PTFE.
Choosing the correct material is the foundational step toward ensuring the accuracy and reproducibility of your electrochemical data.
Summary Table:
| Material | Key Properties | Best For |
|---|---|---|
| High Borosilicate Glass | Chemical stability, thermal shock resistance, transparency | General electrochemistry, visual monitoring |
| PTFE (Teflon) | Outstanding corrosion resistance, inert | Highly corrosive media (e.g., HF, strong bases) |
| Quartz Glass | Superior optical transparency (UV to IR), high thermal stability | Spectroelectrochemistry, UV-initiated reactions |
Optimize Your Electrochemical Experiments with KINTEK
Choosing the right material for your H-type electrolytic cell is critical for achieving accurate and reproducible results. Whether you require the optical clarity of quartz for spectroelectrochemistry or the supreme chemical resistance of PTFE for harsh environments, KINTEK provides high-quality lab equipment tailored to your specific research needs.
Our expertise in laboratory equipment and consumables ensures you get a solution that enhances your experimental integrity. Let us help you select the perfect cell for your application.
Contact KINTEK today to discuss your requirements and elevate your lab's capabilities!
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- What should be observed during an experiment with the H-type electrolytic cell? Key Monitoring for Precise Results
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide
- What preparation steps are needed before starting an experiment with an H-type electrolytic cell? A Guide to Safe and Accurate Results



















