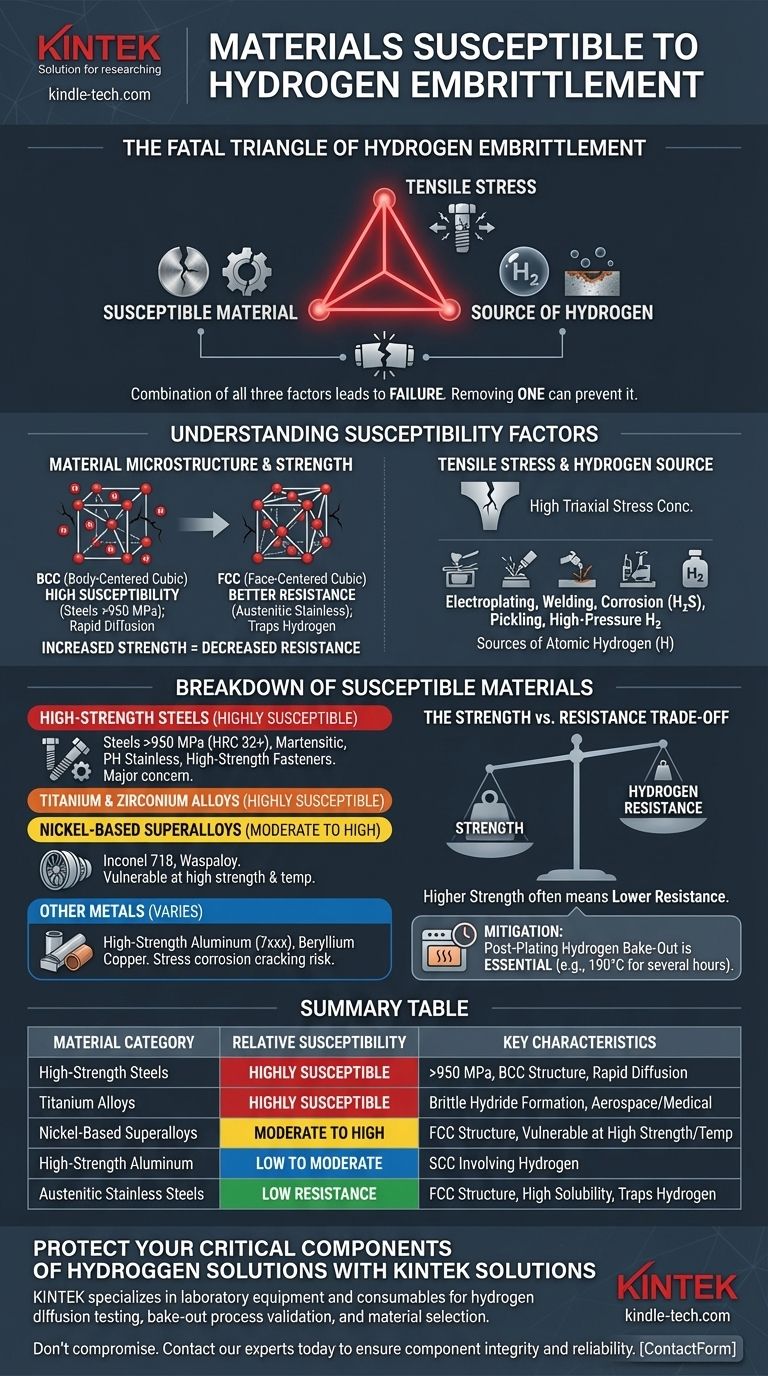
At a high level, the materials most susceptible to hydrogen embrittlement are high-strength metallic alloys. While high-strength steels are the most notorious, the phenomenon also affects critical engineering materials like titanium alloys, nickel-based alloys, and even some high-strength aluminum alloys. The common factor is a combination of high tensile stress, a susceptible microstructure, and a source of atomic hydrogen.
The core principle to understand is that hydrogen embrittlement is not caused by a single factor, but by a "fatal triangle" of conditions: a susceptible material, the presence of tensile stress (either applied or residual), and exposure to a source of hydrogen. Removing any one of these three elements can prevent failure.

Understanding the Key Factors for Susceptibility
Hydrogen embrittlement is a complex failure mechanism. Before listing specific materials, it's crucial to understand why they are susceptible. The risk is dictated by the interplay of the material's internal structure and its external environment.
The Role of Material Microstructure and Strength
A material's internal crystal structure and strength level are the most significant intrinsic factors. Generally, as the strength and hardness of an alloy increase, its resistance to hydrogen embrittlement decreases dramatically.
Materials with a Body-Centered Cubic (BCC) crystal structure, like ferritic and martensitic steels, are highly susceptible. This structure allows for rapid diffusion of small hydrogen atoms but has low solubility, meaning hydrogen doesn't stay "trapped" and can easily move to high-stress regions to initiate cracks.
In contrast, materials with a Face-Centered Cubic (FCC) structure, like austenitic stainless steels (e.g., 304, 316), have much better resistance. The FCC lattice has a higher solubility for hydrogen and a lower diffusion rate, effectively trapping hydrogen atoms in less harmful locations.
The Critical Need for Tensile Stress
Hydrogen atoms migrate to areas of high triaxial tensile stress, such as the tip of a crack, a notch, or even microscopic defects within the material. The stress is the driving force that concentrates hydrogen.
This stress can be from an applied load (e.g., a bolted connection under tension) or from residual stress left over from manufacturing processes like welding, forming, or grinding.
The Essential Source of Hydrogen
A material cannot become embrittled without a source of atomic (H) hydrogen that can be absorbed. This hydrogen can come from numerous sources during manufacturing or service.
Common sources include electroplating, welding with damp electrodes, corrosion (especially in "sour" H₂S environments), cleaning processes like acid pickling, and direct exposure to high-pressure hydrogen gas.
A Breakdown of Susceptible Materials
Based on the principles above, we can categorize materials by their relative susceptibility.
High-Strength Steels (Highly Susceptible)
This is the most widely affected and studied category. Susceptibility becomes a major concern for steels with tensile strengths exceeding 950-1000 MPa (140-145 ksi) or hardness above approximately HRC 32.
Examples include martensitic steels, precipitation-hardening (PH) stainless steels (like 17-4PH in high-strength conditions), and high-strength fasteners (Grade 8 / Class 10.9 and above).
Titanium and Zirconium Alloys (Highly Susceptible)
Titanium alloys, such as the common Ti-6Al-4V, are very prone to hydrogen embrittlement. They can fail via two mechanisms: strain-rate dependent embrittlement from dissolved hydrogen or the formation of brittle titanium hydride phases.
Zirconium alloys, used extensively in the nuclear industry, are also highly susceptible to forming brittle hydrides.
Nickel-Based Superalloys (Moderately to Highly Susceptible)
While their FCC structure provides more resistance than steel, high-strength nickel alloys like Inconel 718 or Waspaloy are susceptible, particularly at high strength levels. Embrittlement is a concern in environments with hydrogen gas, especially at elevated temperatures.
Other Susceptible Metals
- Aluminum Alloys: Generally considered less susceptible, but high-strength 7xxx series alloys can be vulnerable, especially to stress corrosion cracking, which often involves a hydrogen embrittlement mechanism.
- Copper Alloys: Pure copper is resistant, but some high-strength copper alloys like beryllium copper can exhibit susceptibility.
Understanding the Trade-offs: Strength vs. Resistance
When selecting materials, engineers face a fundamental conflict between mechanical properties and environmental resistance.
The Strength-Susceptibility Penalty
The most critical trade-off is strength versus hydrogen resistance. The very processes that make a steel stronger (e.g., quenching and tempering to create a martensitic microstructure) also make it significantly more vulnerable to hydrogen. This is a primary design constraint for high-strength fasteners and structural components.
Processing History Matters
Two components made from the same alloy can have vastly different susceptibilities based on their processing. A component with high residual stress from welding or improper heat treatment will be far more vulnerable than a properly stress-relieved one.
The Importance of Mitigation Steps
For susceptible materials used in hydrogen-charging environments (like plating), mitigation is not optional. A post-plating hydrogen bake-out (e.g., at 190°C / 375°F for several hours) is a standard and necessary procedure to drive absorbed hydrogen out of the part before it can cause damage.
Making the Right Choice for Your Application
Your material selection must be guided by a clear understanding of the service environment and mechanical requirements.
- If your primary focus is maximum strength in a controlled environment: High-strength steels are a valid choice, but you must rigorously control manufacturing processes (plating, welding) and consider post-fabrication baking to eliminate any absorbed hydrogen.
- If your primary focus is reliability in a hydrogen-rich environment (e.g., sour gas): Prioritize inherently resistant materials like qualified nickel alloys, duplex stainless steels, or specific austenitic grades, even if it means accepting a lower strength ceiling.
- If you are balancing strength, weight, and hydrogen exposure (e.g., H2 fuel systems): Materials like austenitic stainless steels (316L) are a common baseline. More advanced applications may require specialized alloys or coatings designed and tested specifically for hydrogen service.
- If you must use a susceptible high-strength fastener: Always specify and verify that a proper post-plating hydrogen embrittlement relief bake has been performed according to standards like ASTM F1941.
Ultimately, preventing hydrogen embrittlement is a matter of proactive design and diligent process control.
Summary Table:
| Material Category | Relative Susceptibility | Key Characteristics |
|---|---|---|
| High-Strength Steels | Highly Susceptible | Susceptible at tensile strengths >950 MPa (HRC 32+); BCC crystal structure allows rapid hydrogen diffusion |
| Titanium Alloys (e.g., Ti-6Al-4V) | Highly Susceptible | Prone to brittle hydride formation; critical in aerospace and medical applications |
| Nickel-Based Superalloys (e.g., Inconel 718) | Moderate to High | FCC structure provides some resistance but vulnerable at high strength levels and elevated temperatures |
| High-Strength Aluminum (7xxx series) | Low to Moderate | Generally resistant but can be vulnerable to stress corrosion cracking involving hydrogen |
| Austenitic Stainless Steels (304, 316) | Low Resistance | FCC structure with high hydrogen solubility provides good inherent resistance |
Protect Your Critical Components from Hydrogen Embrittlement
Hydrogen embrittlement can lead to catastrophic and unexpected failures in high-strength components. At KINTEK, we specialize in providing laboratory equipment and consumables that help you analyze material susceptibility, test for hydrogen diffusion, and implement proper mitigation techniques like hydrogen bake-out processes.
We help you:
- Select the right materials for hydrogen-rich environments
- Validate post-processing treatments to ensure component integrity
- Maintain quality control with reliable lab equipment
Don't let hydrogen embrittlement compromise your projects. Contact our experts today to discuss your specific laboratory needs and find the right solution for your application.
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Filter Testing Machine FPV for Dispersion Properties of Polymers and Pigments
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
People Also Ask
- What is the role of standard sieves in gold scrap leaching kinetic studies? Ensure Precision in Particle Classification
- What are the specifications for test sieves? A Guide to ASTM & ISO Standards for Accurate Particle Analysis
- Why is sieve analysis important? Ensure Consistent Quality and Performance of Your Materials
- How is a vibratory sieve shaker used in the particle size analysis of mechanically alloyed powders? Expert Guide
- What is the function of sieving equipment in CuAlMn alloys? Master Pore Size Precision


















