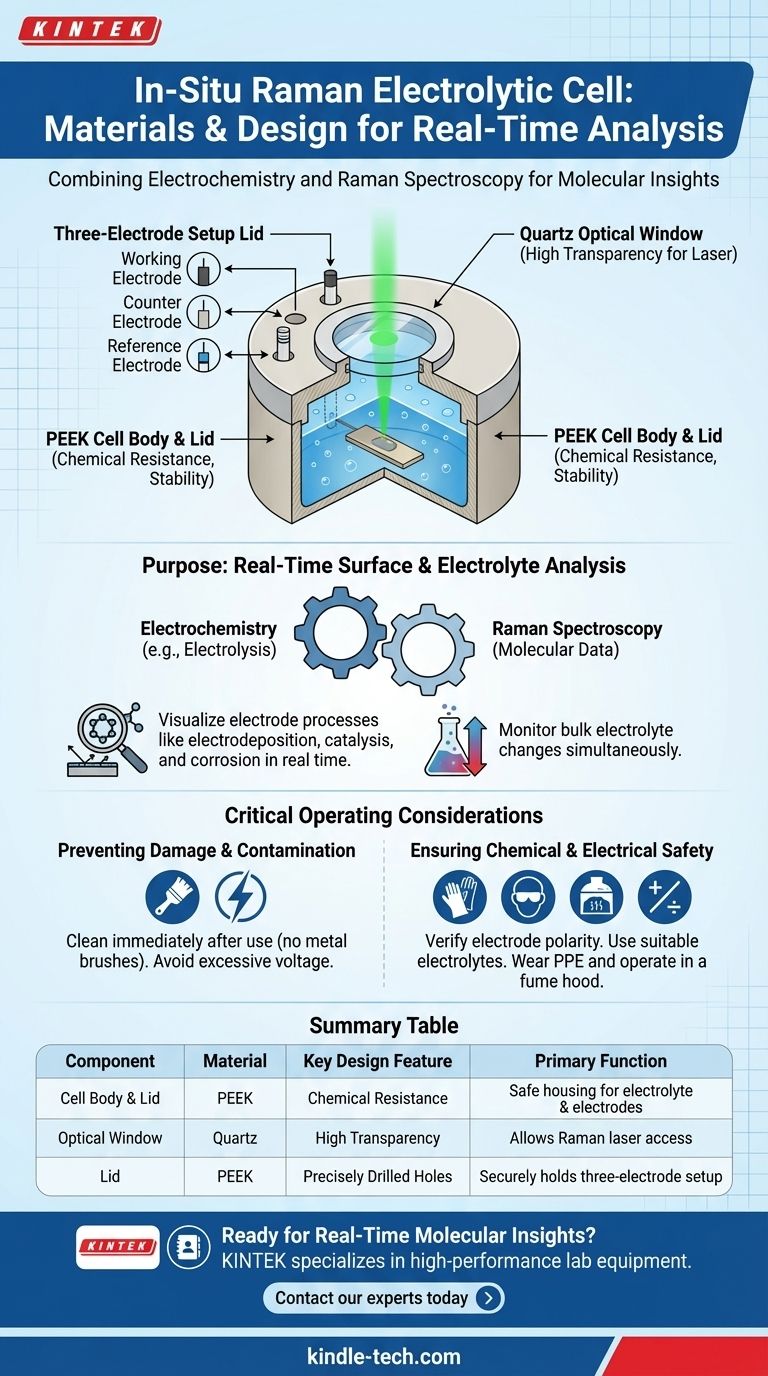
The body and lid of the in-situ Raman electrolytic cell are both constructed from PEEK (Polyetheretherketone). This material is chosen for its excellent chemical resistance and stability. The key design features include a quartz window on the cell body for optical access and a lid precisely drilled for a standard three-electrode electrochemical setup.
This cell is a specialized instrument designed for a single purpose: to enable real-time Raman spectroscopy of an electrode's surface and the surrounding electrolyte during an active electrochemical experiment. Its materials and features are all optimized for chemical inertness and clear optical observation.

The Purpose of an In-Situ Raman Cell
An in-situ Raman electrolytic cell solves a fundamental challenge in electrochemistry: seeing what is happening at the molecular level on an electrode surface in real time.
Combining Two Powerful Techniques
It allows researchers to perform Raman spectroscopy simultaneously with an electrochemical experiment like electrolysis. This provides direct, real-time data on the chemical changes occurring.
Visualizing Electrode Processes
For example, when studying metal electrodeposition, this cell allows for the direct observation of how metal ions are reduced on the electrode surface and how the new deposition layer forms.
Analyzing the Core Components and Materials
The cell's design is a direct reflection of its dual-purpose function, prioritizing both electrochemical integrity and optical clarity.
Cell Body: PEEK and Quartz
The main body is constructed from PEEK, a high-performance thermoplastic. This material is used because it is highly resistant to a wide range of chemicals and solvents used as electrolytes, preventing contamination or degradation of the cell.
A quartz window is integrated into the top of the cell body. This provides a clear, unobstructed path for the Raman laser to focus on the working electrode sample below and collect the scattered light for analysis.
Lid Design: The Three-Electrode System
The lid, also made of PEEK, is designed to securely hold the three essential components of an electrochemical cell.
It features three dedicated holes for the working electrode (the surface of interest), the counter electrode (to complete the circuit), and the reference electrode (to provide a stable potential reference).
Critical Operating Considerations
Due to its specialized and complex construction, proper handling and maintenance are crucial to ensure accurate results and prevent damage.
Preventing Damage and Contamination
Always clean the cell and electrodes immediately after an experiment to prevent residue buildup. When cleaning, avoid using metal brushes that can scratch the electrode or window surfaces.
Be cautious with applied voltage. Excessively high voltage can cause the electrolyte to decompose or damage the electrodes permanently.
Ensuring Chemical and Electrical Safety
Always verify the correct electrode polarity before starting an experiment to avoid reverse connections, which can ruin your sample and experiment.
Choose an electrolyte that is suitable for your specific reaction to prevent unwanted side reactions.
When handling corrosive electrolytes, always wear protective gloves and glasses. It is essential to operate the cell within a fume hood to avoid inhaling any potentially harmful gases generated during electrolysis.
Making the Right Choice for Your Goal
This cell is a powerful tool, but only for the right application. Use these points to determine if it fits your research needs.
- If your primary focus is real-time surface analysis: This cell is ideal for observing phenomena like catalysis, corrosion, or electrodeposition as they happen on the working electrode.
- If your primary focus is monitoring bulk electrolyte changes: The cell is also effective for studying how the composition of the electrolyte solution changes during the electrochemical process.
- If you only need to perform electrochemistry without optical analysis: A simpler, non-optical glass or PEEK electrochemical cell would be a more practical and cost-effective choice.
Ultimately, this specialized cell empowers you to directly correlate electrochemical performance with specific molecular changes.
Summary Table:
| Component | Material | Key Design Feature | Primary Function |
|---|---|---|---|
| Cell Body & Lid | PEEK (Polyetheretherketone) | Excellent chemical resistance and stability | Houses the electrolyte and electrodes safely |
| Optical Window | Quartz | High transparency for laser light | Allows Raman laser access to the working electrode surface |
| Lid | PEEK | Precisely drilled holes | Securely holds the standard three-electrode setup (working, counter, reference) |
Ready to gain real-time molecular insights from your electrochemical experiments?
KINTEK specializes in high-performance lab equipment like the in-situ Raman electrolytic cell. Our expertise ensures you get the right tools for advanced research in catalysis, corrosion, and electrodeposition.
Let's discuss how our solutions can enhance your research: Contact our experts today to find the perfect equipment for your laboratory's needs.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- How should an all-PTFE electrolytic cell be stored after use? Expert Maintenance Tips for Long-Lasting Performance
- What is the technical significance of the aging process in an electrolytic cell? Refine Your Nanotube Structures
- What are the general storage and handling precautions for the electrolysis cell? Protect Your Lab's Precision Equipment
- What are the key maintenance and handling procedures for a thin-layer spectroelectrochemical cell? Protect Your Sensitive Lab Equipment
- What are the typical volumes for an all-PTFE electrolytic cell? Choose the Right Size for Your Experiment
- What should be considered when selecting and using an ion-exchange membrane? A Guide to Optimal Electrochemical Results
- What is the primary benefit of micro-electrochemical cells? Maximize Research with Minimal Reagents
- Why are electrolytic cells essential in titanium production? Powering Circular Efficiency and Cost Savings



















