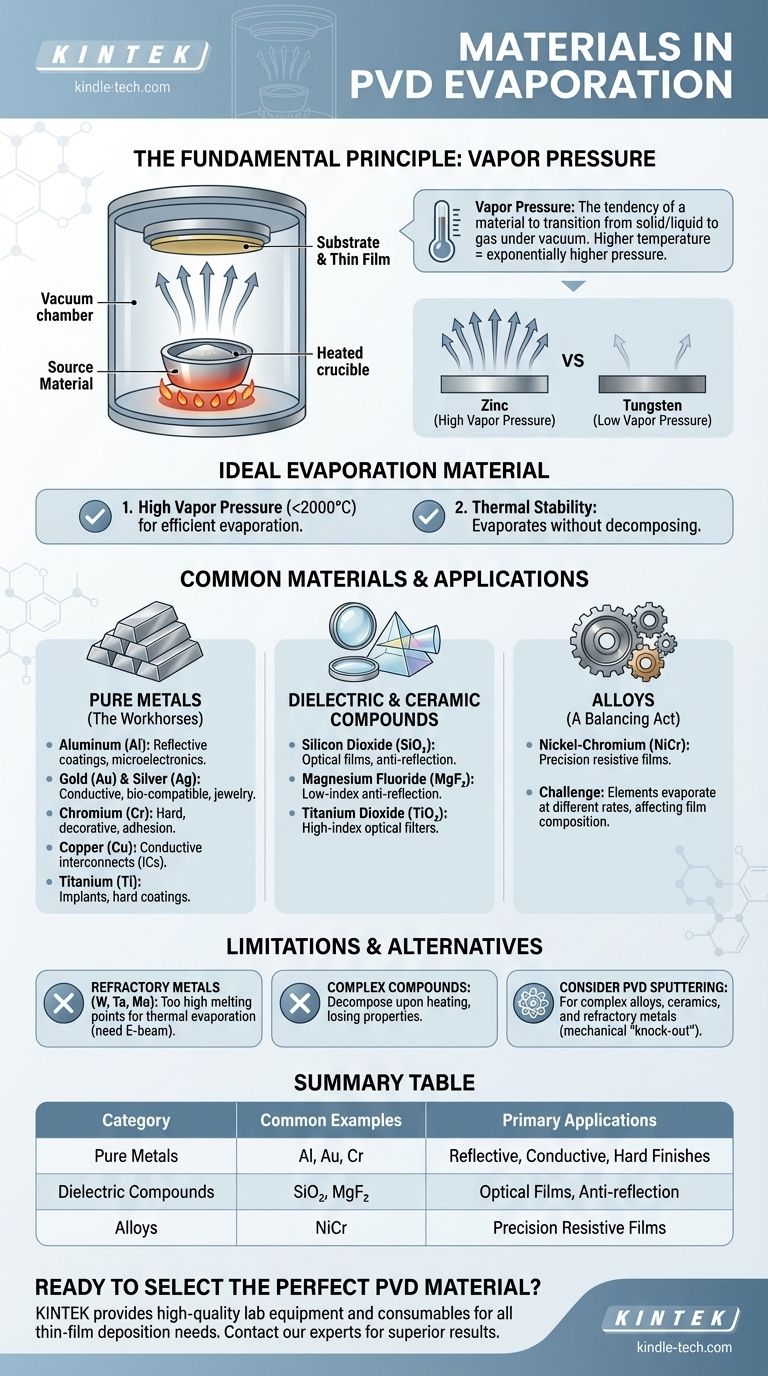
In PVD evaporation, the most common materials used are pure metals and certain dielectric compounds that can be thermally heated to a vapor state without decomposing. Key examples include Aluminum (Al) for reflective coatings, Gold (Au) and Copper (Cu) for conductive layers, Chromium (Cr) for decorative and hard finishes, and Silicon Dioxide (SiO₂) for optical films. The choice is dictated by the material's physical ability to transition into a gas under vacuum.
The critical factor determining if a material is suitable for PVD evaporation is not a fixed list, but its vapor pressure. A material must be able to achieve a high enough vapor pressure at a manageable temperature to evaporate efficiently without breaking down chemically.

The Fundamental Principle: It's All About Vapor Pressure
The evaporation process is governed by a simple physical property. Understanding this is key to selecting the right source material for your coating.
What is Vapor Pressure?
Vapor pressure is the inherent pressure exerted by the vapor of a substance when it is in a closed system at a given temperature. In simpler terms, it’s a measure of a material's tendency to transition from a solid or liquid state into a gas.
Materials with high vapor pressure, like zinc, evaporate easily. Materials with very low vapor pressure, like tungsten, require extremely high temperatures to do so.
How Temperature Drives Evaporation
The PVD evaporation process works by heating a source material in a high-vacuum chamber. As the temperature of the material rises, its vapor pressure increases exponentially.
Once the material's vapor pressure becomes significant, atoms or molecules begin to "boil off" the surface, travel through the vacuum, and condense onto the cooler substrate, forming a thin film.
The Ideal Evaporation Material
An ideal material for thermal evaporation has two main characteristics:
- A high vapor pressure at a reasonably low temperature (e.g., below 2000°C).
- Thermal stability, meaning it evaporates as the intended molecule or atom without decomposing into other substances.
Common Materials Used in PVD Evaporation
Based on the principle of vapor pressure, a specific set of materials has become standard for this process across various industries.
Pure Metals (The Workhorses)
Pure metals are the most straightforward materials to evaporate and are used extensively.
- Aluminum (Al): Widely used for creating highly reflective surfaces for mirrors, decorative coatings, and as a conductive layer in microelectronics.
- Gold (Au) & Silver (Ag): Valued for their superior electrical conductivity, corrosion resistance, and biocompatibility. Used in electronics, medical devices, and jewelry.
- Chromium (Cr): Provides a hard, corrosion-resistant, and bright decorative finish. It's also an excellent adhesion layer for other metals.
- Titanium (Ti): Used for biocompatible implants, hard coatings (often with nitrogen to form TiN), and as an adhesion layer.
- Copper (Cu): A primary material for conductive interconnects in integrated circuits and printed circuit boards.
Dielectric & Ceramic Compounds
Evaporating compounds is more complex, but essential for optical applications.
- Silicon Monoxide (SiO) & Dioxide (SiO₂): Used extensively in optics to create protective layers and modify refractive index for anti-reflection coatings.
- Magnesium Fluoride (MgF₂): A classic low-index material for anti-reflection lens coatings.
- Titanium Dioxide (TiO₂): A high-index optical material used in multilayer interference filters.
Alloys (A Balancing Act)
Evaporating alloys can be challenging. Each element in the alloy has its own unique vapor pressure, meaning the element with the higher vapor pressure will evaporate faster.
This can cause the composition of the vapor—and therefore the final thin film—to differ from the source material. However, some alloys like Nickel-Chromium (NiCr) are commonly evaporated to create precision resistive films.
Understanding the Trade-offs: Evaporation's Limitations
No single process is perfect for every material or application. Knowing the limits of evaporation is crucial for making an informed decision.
The Challenge of Refractory Metals
Metals with extremely high melting points and low vapor pressures, such as Tungsten (W), Tantalum (Ta), and Molybdenum (Mo), are very difficult to deposit with thermal evaporation. They require immense energy, often demanding more advanced techniques like electron-beam evaporation.
When Compounds Decompose
Many complex compounds and polymers cannot be thermally evaporated. When heated, their chemical bonds break before they achieve a sufficient vapor pressure, causing them to decompose. The resulting film would not have the desired chemical structure or properties.
When to Consider Sputtering
For materials that are difficult to evaporate—including most complex alloys, ceramics, and refractory metals—PVD sputtering is often the superior choice. Sputtering is a mechanical "knock-out" process, not a thermal one, allowing it to deposit virtually any material while maintaining the source's original composition.
Making the Right Choice for Your Application
Your final material choice depends entirely on the properties you need in the final film.
- If your primary focus is high reflectivity or conductivity: Your best candidates are pure metals like Aluminum, Silver, Gold, or Copper.
- If your primary focus is a hard, decorative, or protective finish: Chromium is an excellent and common choice for direct evaporation.
- If your primary focus is an optical coating: You will need to use dielectric compounds like Silicon Dioxide (SiO₂) or Magnesium Fluoride (MgF₂).
- If your primary focus is depositing a complex alloy or refractory metal: Evaporation may be unsuitable; you should strongly consider PVD sputtering for better compositional control.
Ultimately, selecting the right material is a process of matching the desired film properties with the physical realities of the PVD method you intend to use.
Summary Table:
| Material Category | Common Examples | Primary Applications |
|---|---|---|
| Pure Metals | Aluminum (Al), Gold (Au), Chromium (Cr) | Reflective coatings, conductive layers, hard finishes |
| Dielectric Compounds | Silicon Dioxide (SiO₂), Magnesium Fluoride (MgF₂) | Optical films, anti-reflection coatings |
| Alloys | Nickel-Chromium (NiCr) | Precision resistive films |
Ready to select the perfect PVD evaporation material for your specific application?
KINTEK specializes in providing high-quality lab equipment and consumables for all your thin-film deposition needs. Whether you are working with pure metals for conductive layers or dielectric compounds for optical coatings, our expertise ensures you get the right materials and equipment for superior results.
Contact our experts today to discuss your project and discover how KINTEK can enhance your laboratory's capabilities.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Custom PTFE Teflon Parts Manufacturer for Culture Dish and Evaporation Dish
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Vacuum Cold Trap Direct Cold Trap Chiller
People Also Ask
- Why does PECVD commonly use RF power input? For Precise Low-Temperature Thin Film Deposition
- How are PECVD and CVD different? A Guide to Choosing the Right Thin-Film Deposition Process
- Why is PECVD environment friendly? Understanding the Eco-Friendly Benefits of Plasma-Enhanced Coating
- What are the advantages of PECVD? Enable Low-Temperature, High-Quality Thin-Film Deposition
- What are the applications of PECVD? Essential for Semiconductors, MEMS, and Solar Cells



















