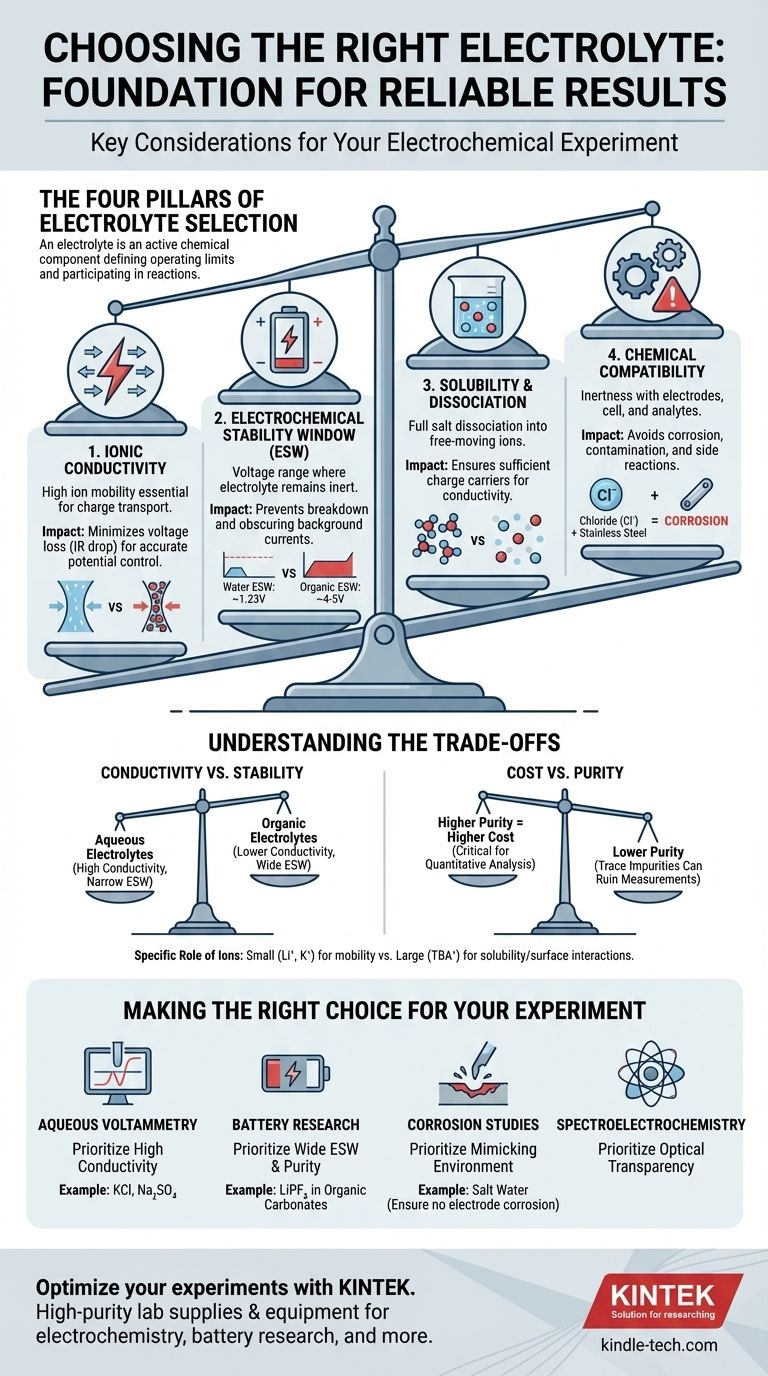
When selecting an electrolyte for an experiment, you must evaluate four key properties: its ionic conductivity, its electrochemical stability window, its solubility in the chosen solvent, and its chemical compatibility with your entire experimental system. These factors collectively determine whether the electrolyte will support the reaction you want to study or introduce artifacts that invalidate your results.
The central takeaway is that an electrolyte is not merely a passive conductor. It is an active chemical component that defines the operating limits of your electrochemical cell and can directly participate in side reactions, making its careful selection foundational to obtaining reliable and meaningful data.
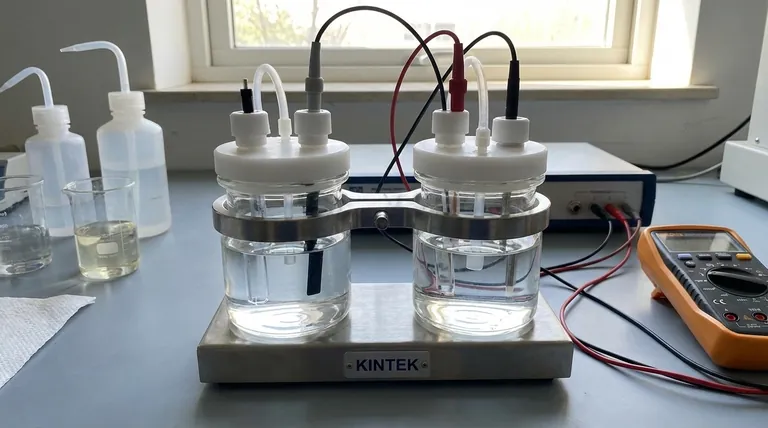
The Four Pillars of Electrolyte Selection
Choosing the right electrolyte is a process of balancing requirements. The ideal electrolyte facilitates your desired reaction while remaining completely inert and invisible to the measurement. We can break down this selection process into four critical considerations.
Pillar 1: Ionic Conductivity
An electrolyte's primary job is to transport charge between the electrodes. High ionic conductivity is essential for this.
Poor conductivity leads to a large ohmic drop (or IR drop), which is a voltage loss across the electrolyte. This means the potential you apply to your cell is not the potential actually experienced at the electrode surface, leading to inaccurate measurements of reaction potentials.
Think of the electrolyte as a highway for ions. A high-conductivity electrolyte is a wide, clear superhighway, while a low-conductivity one is a congested side street, slowing everything down and wasting energy.
Pillar 2: Electrochemical Stability Window (ESW)
The electrochemical stability window (ESW) is the range of potentials where the electrolyte itself—both the salt and the solvent—does not get oxidized or reduced.
Operating outside this window is a critical failure. If you apply a potential that is too positive or too negative, you will start breaking down the electrolyte instead of studying your analyte. This creates large background currents that can completely obscure the signal you are trying to measure.
For example, the ESW of water is only about 1.23 V. For experiments requiring higher potentials, like in lithium-ion battery research, you must switch to organic solvents and specific salts (e.g., LiPF₆ in organic carbonates) that offer a much wider window (~4-5 V).
Pillar 3: Solubility and Dissociation
For an electrolyte to function, the salt must not only dissolve but also fully dissociate into free-moving cations and anions in the solvent.
If the salt has poor solubility or forms tight "ion pairs" instead of separating, the number of available charge carriers decreases dramatically. This directly lowers the ionic conductivity, undermining the electrolyte's primary function.
Always ensure your chosen salt is highly soluble in your chosen solvent at the concentration you intend to use (typically 0.1 M to 1.0 M for lab-scale experiments).
Pillar 4: Chemical Compatibility
The electrolyte must be chemically inert with respect to every component in your cell: the working electrode, counter electrode, reference electrode, and the cell body itself.
A common mistake is using an electrolyte containing chloride ions (like KCl) with stainless steel components. Chloride is highly corrosive to stainless steel and will cause pitting and release metal ions into your solution, contaminating the experiment.
Similarly, the electrolyte's ions should not react with or adsorb too strongly onto your electrode surface unless that interaction is the specific phenomenon you are studying.
Understanding the Trade-offs
There is no single "best" electrolyte; every choice involves balancing competing factors. Understanding these trade-offs is the mark of an experienced researcher.
Conductivity vs. Stability
Aqueous electrolytes (e.g., NaCl in water) offer excellent ionic conductivity but have a very narrow electrochemical stability window. Conversely, many organic solvent-based electrolytes provide a wide stability window but often suffer from lower conductivity. Your choice depends on whether your experiment is limited by voltage or current efficiency.
Cost vs. Purity
High-purity, "battery grade" or "electrochemical grade" salts and solvents are significantly more expensive. However, trace impurities (like water in a non-aqueous electrolyte or halide ions) can introduce unwanted electrochemical signals and ruin sensitive measurements. For simple demonstrations, a lower grade may suffice, but for quantitative analysis, investing in purity is critical.
The Specific Role of Ions
Not all ions are created equal. Small, mobile ions like lithium (Li⁺) or potassium (K⁺) are excellent charge carriers. However, large organic ions like tetrabutylammonium (TBA⁺) are often used to increase the solubility of nonpolar analytes in polar solvents and can help minimize unwanted interactions at the electrode surface.
Making the Right Choice for Your Experiment
Your specific goal dictates which properties to prioritize.
- If your primary focus is standard aqueous voltammetry: Prioritize high conductivity and use a simple, inert salt like potassium chloride (KCl) or sodium sulfate (Na₂SO₄), ensuring your potential range stays within water's stability window.
- If your primary focus is high-voltage battery research: Prioritize a wide electrochemical stability window and extreme purity, typically using salts like LiPF₆ or LiClO₄ in a mixture of organic carbonate solvents.
- If your primary focus is corrosion studies: Prioritize creating an electrolyte that accurately mimics the real-world environment (e.g., salt water), while ensuring it does not corrode your reference or counter electrodes.
- If your primary focus is spectroelectrochemistry: Prioritize optical transparency of both the salt and the solvent in your desired wavelength range, in addition to the standard electrochemical requirements.
Ultimately, the electrolyte you choose sets the stage and defines the rules for your entire electrochemical measurement.
Summary Table:
| Selection Pillar | Key Consideration | Impact on Experiment |
|---|---|---|
| Ionic Conductivity | High ion mobility | Minimizes voltage loss (IR drop) for accurate potential control. |
| Electrochemical Stability Window (ESW) | Voltage range of stability | Prevents electrolyte breakdown and obscuring background currents. |
| Solubility & Dissociation | Full salt dissociation into ions | Ensures sufficient charge carriers for effective conductivity. |
| Chemical Compatibility | Inertness with cell components | Avoids corrosion, contamination, and unwanted side reactions. |
Ready to optimize your electrochemical experiments with the right lab equipment and consumables? The correct electrolyte is just one part of the equation. KINTEK specializes in providing high-purity lab supplies and equipment tailored for electrochemistry, battery research, corrosion studies, and more. Our products help you achieve the precise control and reliable data your work demands.
Contact our experts today to discuss your specific application needs and discover how KINTEK can support your laboratory's success.
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Thin-Layer Spectral Electrolysis Electrochemical Cell
People Also Ask
- What is a H type cell? A Guide to Divided Electrochemical Cells for Accurate Experiments
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- What is the structure of an H-type exchangeable membrane electrolytic cell? A Guide to Precise Electrochemical Separation
- What optical features does the H-type electrolytic cell have? Precision Quartz Windows for Photoelectrochemistry
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control



















