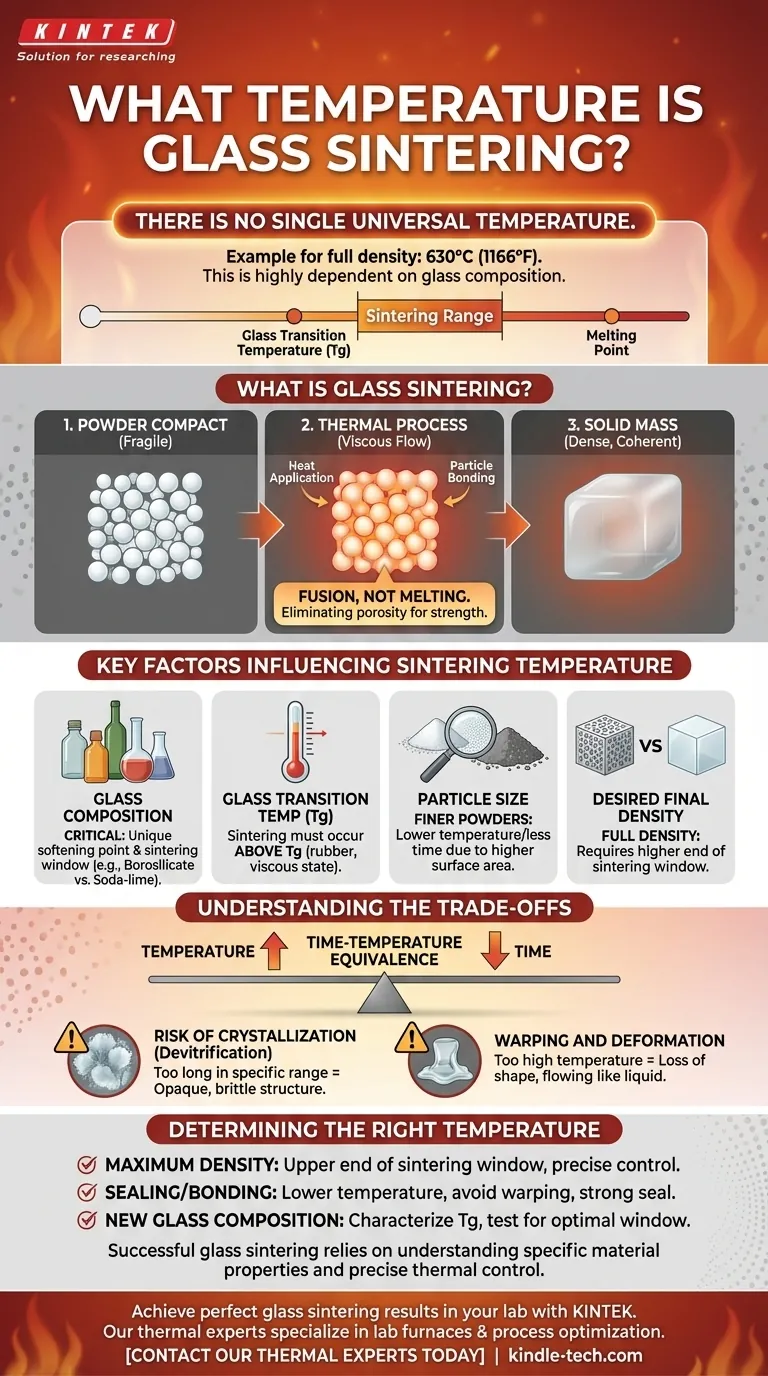
While there is no single universal temperature, a specific example of glass sintering to achieve full density is 630°C (1166°F). This temperature, however, is highly dependent on the specific type of glass being used. The process occurs within a carefully controlled temperature window unique to each glass composition.
The critical takeaway is that glass sintering is not about a fixed temperature but a process that occurs within a specific range: above the glass's transition temperature (where it softens) but below its melting point. The exact temperature is a function of the glass chemistry and the desired final density.

What is Glass Sintering?
Sintering is a thermal process used to densify a powder compact, turning it into a solid, coherent mass. It's a foundational technique in ceramics and materials science.
A Process of Fusion, Not Melting
Imagine packing snow together to make a snowball. The individual flakes, under pressure, begin to bond. Sintering is the thermal equivalent of this.
Instead of fully melting the glass into a liquid, the material is heated just enough for the surfaces of the glass particles to become viscous and fuse together. The particles stick, the gaps (or pores) between them shrink, and the material becomes dense.
The Goal: Eliminating Porosity
The primary goal of sintering is to remove the empty space between the initial glass particles. This transforms a fragile powder compact into a strong, non-porous, and often transparent or translucent final part.
Key Factors Influencing Sintering Temperature
The 630°C figure is a single data point for a specific situation. In practice, the ideal temperature is determined by several interconnected variables.
Glass Composition is Critical
This is the most important factor. A soda-lime glass (used in bottles and windows) will have a very different sintering window than a borosilicate glass (like Pyrex) or a specialized sealing glass. Each formulation has a unique softening point.
Glass Transition Temperature (Tg)
Every glass has a glass transition temperature (Tg), the point where it changes from a hard, brittle solid to a rubbery, viscous state. Sintering must occur above the Tg, as this is the point where the glass is soft enough for particles to flow and fuse.
Particle Size
Finer glass powders, with their higher relative surface area, will typically sinter at a slightly lower temperature or in less time compared to coarser powders. More surface area provides more points of contact for fusion to begin.
Desired Final Density
Achieving partial density for a porous filter requires a different temperature and time than achieving the "full density" mentioned in the reference. Full densification, where virtually all pores are eliminated, generally requires operating at the higher end of the sintering window.
Understanding the Trade-offs
Selecting a sintering temperature is a balancing act. Pushing the temperature too high or holding it for too long can introduce significant problems.
Temperature vs. Time
You can often achieve a similar level of densification by using a lower temperature for a longer duration or a higher temperature for a shorter duration. This is known as the time-temperature equivalence.
The Risk of Crystallization (Devitrification)
This is the most critical pitfall. If glass is held for too long within a certain temperature range, its amorphous (disordered) atomic structure can begin to rearrange into an ordered, crystalline structure. This process, called devitrification, can make the glass opaque, brittle, and weak.
Warping and Deformation
If the temperature is too high, the viscosity of the glass drops too much. Instead of just fusing, the entire part will begin to flow like a thick liquid, losing its intended shape and dimensions. Precise temperature control is essential to sinter the material without causing it to slump.
Determining the Right Temperature for Your Application
The correct approach depends entirely on your material and your final goal.
- If your primary focus is achieving maximum density: You will need to operate near the upper end of the sintering window for that specific glass, requiring precise control to get close to the deformation point without causing crystallization.
- If your primary focus is sealing or bonding: A lower temperature within the sintering range may be sufficient to create a strong hermetic seal without risking damage or warping of the components being joined.
- If your primary focus is working with a new glass composition: You must first characterize the material's glass transition temperature (Tg) and then perform a series of tests at increasing temperatures to identify the optimal window between sintering and unwanted deformation.
Ultimately, successful glass sintering relies on understanding your material's specific properties and precisely controlling the thermal cycle.
Summary Table:
| Factor | Influence on Sintering Temperature |
|---|---|
| Glass Composition | Most critical factor; each type (e.g., borosilicate) has a unique sintering window. |
| Glass Transition Temp (Tg) | Sintering must occur above this temperature for particles to fuse. |
| Particle Size | Finer powders can sinter at slightly lower temperatures due to higher surface area. |
| Desired Final Density | Full density requires higher temperatures within the sintering window. |
Achieve perfect glass sintering results in your lab. The precise temperature is critical to avoid warping, crystallization, or incomplete densification. KINTEK specializes in lab furnaces and thermal processing expertise for laboratory needs. Our team can help you select the right equipment and parameters for your specific glass composition and application goals.
Contact our thermal experts today to optimize your sintering process!
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- Multi-zone Laboratory Tube Furnace
People Also Ask
- What is the difference between a furnace and oven? Understanding Their Unique Heating Purposes
- What is the heat capacity of a muffle furnace? Understanding Thermal Mass for Optimal Performance
- What is the difference between sintering and vitrification? Key Thermal Process Distinctions
- How do you check the temperature of a muffle furnace? A Guide to Precise Monitoring
- What is a furnace used in the lab? Your Guide to High-Temperature Precision



















