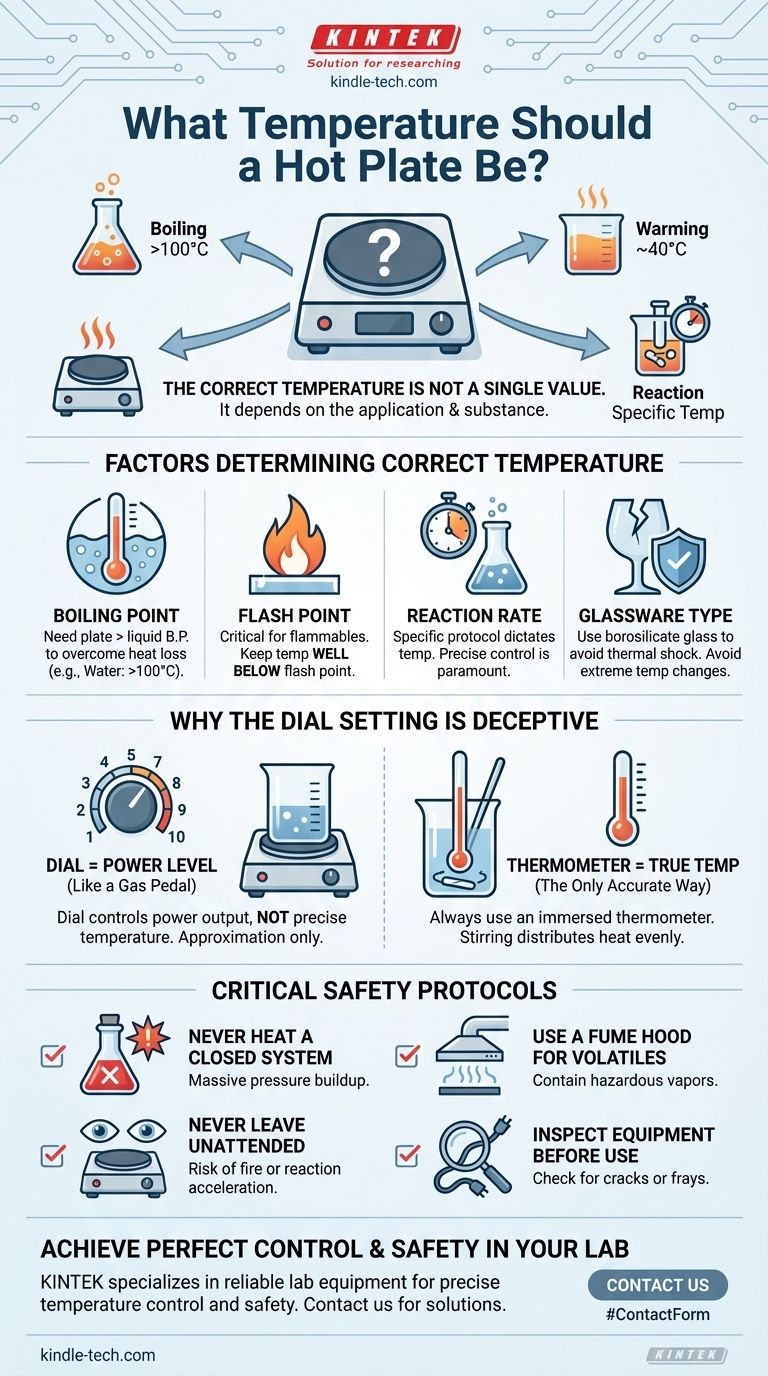
The correct temperature for a hot plate is not a single value; it is entirely dependent on the specific application and the substance you are heating. For example, boiling water requires a plate surface temperature above 100°C (212°F), while gently warming a sensitive chemical solution might only require 40°C (104°F). The key is to match the temperature to the boiling point, melting point, or required reaction temperature of your specific material.
The most critical mistake is assuming the temperature dial on a hot plate accurately reflects the temperature of your liquid. The dial controls power output, not the precise temperature of your sample. Always use a separate, properly placed thermometer to measure the actual temperature of the substance you are heating.

Factors That Determine the Correct Temperature
The "right" setting is a function of your specific goal and the chemical properties of the substance you are working with. You must consider several factors to operate safely and effectively.
The Boiling Point of Your Liquid
The most common use for a hot plate is to boil liquids. To achieve this, the surface of the hot plate must be significantly hotter than the liquid's boiling point to overcome heat loss to the environment.
For water, which boils at 100°C (212°F) at sea level, you will need a plate setting that brings the liquid to that temperature and sustains it.
The Flash Point of Your Substance
For flammable liquids, the flash point is the most critical safety parameter. This is the lowest temperature at which a liquid can form an ignitable vapor mixture in the air.
Heating a liquid near or above its flash point using a hot plate with an open heating element creates a severe fire and explosion hazard. Always know the flash point of your chemicals and keep the temperature well below it.
The Desired Reaction Rate
In chemistry, temperature directly influences the rate of a reaction. A specific procedure or protocol will dictate the precise temperature needed.
Exceeding this temperature can accelerate the reaction uncontrollably, cause unwanted side reactions, or lead to the decomposition of your product. Precise control is paramount.
The Type of Glassware
Standard glass can shatter from thermal shock—a rapid change in temperature. Always use borosilicate glass (branded as Pyrex or KIMAX) for laboratory heating.
Even with borosilicate glass, avoid extreme temperature changes, like placing a hot beaker on a cold surface.
Why the Dial Setting Is Deceptive
Trusting your hot plate's dial is a common and dangerous mistake. The number on the dial is an approximation of power, not a guarantee of temperature.
The Dial Is a Power Level, Not a Thermometer
Think of the dial like a gas pedal, not a speedometer. A setting of "5" does not mean "50°C." It means the plate is receiving a certain amount of electrical power.
The final temperature of your liquid depends on the volume of the liquid, the size and shape of the container, and the ambient room temperature.
The Critical Role of a Thermometer
The only way to know the true temperature of your sample is to measure it directly. Use a thermometer or thermocouple immersed in the liquid (but not touching the bottom of the container) for an accurate reading.
Heat Distribution and Stirring
A liquid heated from the bottom is not uniform in temperature. The bottom layer can be significantly hotter than the top, a phenomenon known as thermal stratification.
Using a magnetic stirrer and a stir bar is essential for most applications. Stirring distributes the heat evenly, prevents dangerous "bumping" or boil-overs, and ensures your thermometer reading reflects the true average temperature of the solution.
Critical Safety Protocols to Follow
Mishandling a hot plate can lead to serious injury or fire. Adhering to these rules is non-negotiable.
Never Heat a Closed System
Heating a liquid in a sealed or closed container will cause a massive pressure buildup as the liquid vaporizes. This can turn your glassware into a bomb. Always ensure the system is open to the atmosphere or properly vented.
Use a Fume Hood for Volatiles
When working with flammable, toxic, or strong-smelling substances, you must perform the heating inside a fume hood. This contains and exhausts any hazardous vapors that are generated.
Never Leave a Hot Plate Unattended
A reaction can accelerate unexpectedly, or a liquid can boil dry, creating a fire hazard. Stay present and attentive for the entire duration of the heating process.
Inspect Your Equipment Before Use
Before plugging it in, check the hot plate's power cord for cracks or frays. Inspect your glassware for any cracks or chips that could cause it to fail under thermal stress.
Setting the Temperature for Your Task
Use the following guidelines to determine the best approach for your specific goal.
- If your primary focus is boiling a non-flammable liquid (e.g., water): Start with a medium-high setting until boiling begins, then reduce the power to maintain a gentle, controlled boil, using a thermometer to monitor.
- If your primary focus is heating a flammable solvent: Work in a fume hood, use a water bath as a thermal buffer, and set the hot plate temperature well below the solvent's flash point, confirming with an immersed thermometer.
- If your primary focus is running a specific chemical reaction: Strictly follow the temperature specified in your protocol, using a thermometer and magnetic stir bar for precise and uniform control.
- If your primary focus is keeping a solution warm: Use the lowest possible setting that maintains the desired temperature and verify it periodically with a thermometer.
Ultimately, precise and safe heating is achieved not by trusting the dial, but by directly measuring your sample and understanding its fundamental properties.
Summary Table:
| Factor | Why It Matters | Key Consideration |
|---|---|---|
| Boiling Point | To effectively boil a liquid | Set plate temperature significantly above the liquid's boiling point |
| Flash Point | Critical for safety with flammable liquids | Always keep temperature well below the flash point |
| Reaction Rate | For controlled chemical processes | Follow protocol specifications precisely |
| Glassware Type | Prevents thermal shock and breakage | Use borosilicate glass (e.g., Pyrex) |
Achieve Perfect Control and Safety in Your Lab
Setting the right hot plate temperature is crucial for both the success of your experiments and the safety of your team. Don't leave it to guesswork.
KINTEK specializes in reliable lab equipment and consumables designed for precise temperature control and operator safety. Whether you need a hot plate with a magnetic stirrer, accurate thermometers, or durable borosilicate glassware, we have the solutions to meet your laboratory's specific needs.
Contact us today to find the right equipment for your application. Our experts are ready to help you enhance your lab's efficiency and safety.
#ContactForm to get a personalized consultation!
Visual Guide
Related Products
- Laboratory Small Constant Temperature Heated Magnetic Stirrer Heater and Stirrer
- Double Plate Heating Press Mold for Lab
- High Performance Laboratory Stirrers for Diverse Applications
- Automatic Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
- Heated Hydraulic Press Machine with Heated Plates Split Manual Laboratory Hot Press
People Also Ask
- Why is a precision temperature-controlled heating stage used for CsPbBr3 annealing? Achieve High-Efficiency Films
- What is the primary purpose of using the Hot Press process for Thallium Bromide? Achieve High-Performance TlBr Crystals
- What are the advantages of using a laboratory hydraulic press for hot pressing? Achieve Peak Nano-Composite Density
- What role does a high-precision hot plate play in N-CXG synthesis? Achieve Perfect Precursor Homogenization
- What is the process of pressing sintering? A Guide to Powder Metallurgy & Ceramics Fabrication
- What is the purpose of a hot press? Transform Materials with Heat and Pressure
- What is hot press vs cold press machine? Choose the Right Method for Your Materials
- What is the process of a hot press machine? Bond, Shape, and Densify Materials with Precision



















