At its core, a controlled atmosphere uses a specific, engineered gas environment to achieve a desired outcome during heat treatment. The most common gases are nitrogen, argon, hydrogen, and sometimes oxygen, which are used individually or in mixtures to displace ambient air and control the chemical reactions that occur on a material's surface at high temperatures.
The fundamental choice of gas comes down to a simple question: are you trying to prevent a chemical reaction or cause a specific one? Gases are selected for being either chemically inert to protect the material or strategically reactive to modify it.
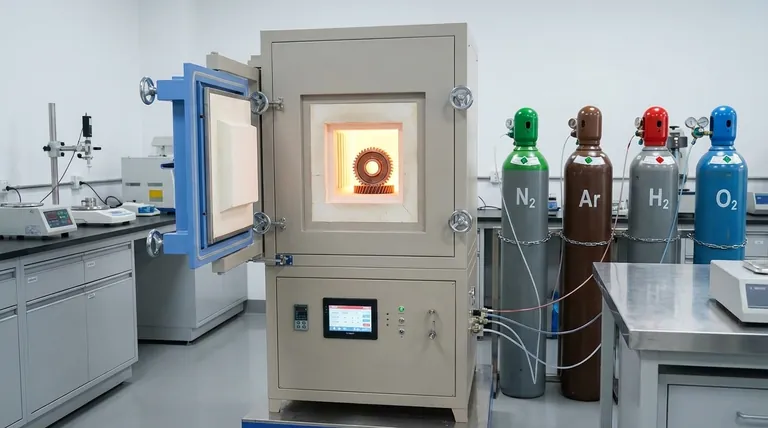
The Purpose of a Controlled Atmosphere
The primary goal of controlling a furnace's atmosphere is to manage chemical reactions, most notably oxidation. When heated in the presence of air, most metals will readily react with oxygen, forming oxides on the surface.
Preventing Unwanted Reactions
This oxidation often manifests as undesirable scaling, discoloration, or a change in the material's surface properties.
By replacing the air (which is roughly 78% nitrogen, 21% oxygen) with a controlled gas, you can prevent these reactions, ensuring the material exits the furnace in a clean, bright, and unaltered state.
Promoting Desired Reactions
Conversely, some processes require a specific reaction. A reactive gas can be introduced to clean the material's surface, bond specific elements to it, or create a controlled, protective oxide layer.
Common Gases and Their Functions
The selection of a gas is dictated entirely by its chemical properties and its interaction with the material being processed.
Inert Gases (The Protectors)
Inert gases are non-reactive and serve as a stable "blanket" to protect the material from oxygen and other contaminants.
Nitrogen (N₂) Nitrogen is the most widely used carrier gas due to its relative inertness and low cost. It effectively displaces oxygen, making it ideal for general-purpose heat treating of many common metals.
Argon (Ar) Argon is a true noble gas, meaning it is more inert than nitrogen. It is used for materials that are highly sensitive or can react with nitrogen at high temperatures, such as titanium and certain stainless steels.
Reactive Gases (The Modifiers)
Reactive gases are chosen to intentionally cause a chemical change on the material's surface.
Hydrogen (H₂) Hydrogen is a powerful reducing agent. This means it actively strips oxygen from metal oxides that may already be on the material's surface. A small percentage of hydrogen is often mixed with nitrogen to produce a clean, bright finish.
Oxygen (O₂) Oxygen is used when the goal is controlled oxidation. This may be done to create a specific, protective oxide layer on a material for passivation or to achieve a particular aesthetic finish.
Understanding the Trade-offs
Choosing an atmosphere is a balance of process requirements, material compatibility, cost, and safety.
Purity vs. Cost
Nitrogen is significantly less expensive than Argon. For most applications involving steel and copper alloys, nitrogen provides a sufficient protective atmosphere. The higher cost of Argon is only justified when processing materials that would be compromised by nitrogen.
Reactivity vs. Safety
Hydrogen is highly effective for cleaning and brightening but is also flammable. Using hydrogen, even in small percentages mixed with nitrogen, requires stringent safety protocols, specialized equipment, and proper ventilation to mitigate the risk of explosion.
Material Compatibility
The gas must be compatible with the workpiece. For example, using a nitrogen-based atmosphere to process titanium can cause nitrogen to bond with the metal, forming titanium nitrides and making the surface brittle. This is a scenario where paying the premium for Argon is essential.
Selecting the Right Atmosphere for Your Process
Your choice of gas should be a direct reflection of your end goal for the material.
- If your primary focus is cost-effective oxidation prevention for common metals: A pure Nitrogen atmosphere is almost always the correct choice.
- If your primary focus is achieving a bright, clean surface on metals like steel or copper: A Nitrogen-Hydrogen mixture provides the best balance of cost and performance.
- If your primary focus is processing highly reactive or exotic materials (like titanium or refractory metals): A pure Argon or Argon-Hydrogen atmosphere is required to prevent unwanted reactions.
Ultimately, mastering a controlled atmosphere is about precisely directing the chemistry inside your furnace to achieve your desired material properties.
Summary Table:
| Gas | Type | Primary Function | Common Uses |
|---|---|---|---|
| Nitrogen (N₂) | Inert | Cost-effective oxidation prevention | General-purpose heat treating of steel and copper alloys |
| Argon (Ar) | Inert (Noble) | Maximum protection for sensitive materials | Processing titanium and certain stainless steels |
| Hydrogen (H₂) | Reactive | Reducing agent for a bright, clean finish | Mixed with nitrogen for surface cleaning |
| Oxygen (O₂) | Reactive | Controlled oxidation for specific surface layers | Passivation and aesthetic finishing |
Ready to Optimize Your Heat Treatment Process?
Choosing the right controlled atmosphere is critical for achieving your desired material properties, from preventing oxidation to creating a perfect surface finish. KINTEK specializes in providing the lab equipment and expert support you need to master your furnace's chemistry.
We supply high-purity gases and reliable furnace systems tailored to your specific application, whether you're working with common alloys or exotic materials. Our team can help you balance performance, cost, and safety to ensure optimal results.
Contact us today to discuss your controlled atmosphere requirements and discover how KINTEK can enhance your laboratory's capabilities. Get in touch via our contact form for a personalized consultation.
Visual Guide

Related Products
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- Can nitrogen gas be heated? Leverage Inert Heat for Precision and Safety
- What is meant by inert atmosphere? A Guide to Preventing Oxidation & Ensuring Safety
- What is the role of an atmosphere-controlled tube furnace in Cu-Mo sintering? Achieve High-Purity Densification
- How does an atmosphere furnace facilitate the post-treatment of nickel-plated carbon fibers? Ensure Peak Bonding
- How we can develop inert atmosphere for a chemical reaction? Master Precise Atmospheric Control for Your Lab



















