In short, metals that cannot be hardened by conventional heat treatment are those lacking a specific internal mechanism for structural change, such as allotropic phase transformation or precipitation. This primarily includes low-carbon steels (with less than 0.3% carbon), austenitic stainless steels (like 304 or 316), and most common non-ferrous metals in their pure or simple alloyed forms, such as pure copper, pure aluminum, or nickel alloys.
The ability to harden a metal through heat treatment is not an inherent property of all metals. It depends entirely on whether the metal's atomic structure can be intentionally manipulated by a thermal cycle to create a new, harder, more stressed internal phase.

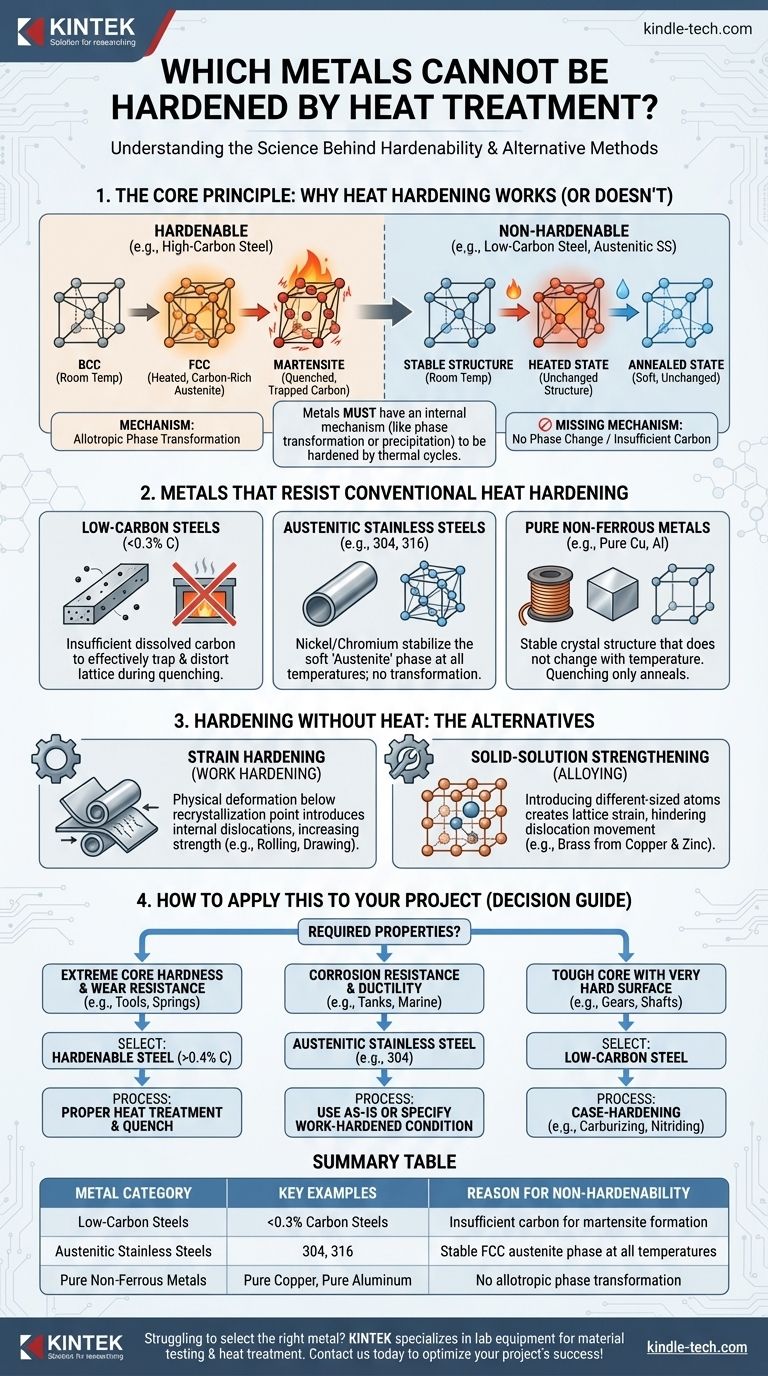
The Core Principle: Why Heat Hardening Works
To understand which metals cannot be hardened, we must first understand the mechanism that makes hardening possible. It is not the heat itself that hardens the metal, but the structural transformation the heat enables.
Allotropic Phase Transformation
The most common hardening mechanism applies to carbon steels. It relies on allotropy—the ability of an element to exist in different crystal structures at different temperatures.
Iron transforms from a Body-Centered Cubic (BCC) structure at room temperature to a Face-Centered Cubic (FCC) structure, called austenite, when heated above a critical temperature. The FCC structure can dissolve significantly more carbon than the BCC structure.
When this carbon-rich austenite is cooled rapidly (a process called quenching), the carbon atoms are trapped. The iron tries to return to its BCC form but is distorted by the trapped carbon, creating a new, highly strained, and very hard structure called martensite.
Precipitation Hardening (Age Hardening)
This is a different mechanism common in certain non-ferrous alloys, such as specific aluminum, copper, and nickel alloys.
In this process, the metal is heated to dissolve alloying elements into a uniform solid solution and then quenched. A subsequent, lower-temperature heating cycle (aging) causes these elements to precipitate out of the solution as extremely small, hard particles within the metal's crystal lattice. These particles obstruct internal movement, thereby increasing the material's hardness and strength.
Metals That Resist Conventional Heat Hardening
If a metal lacks the ability to undergo one of these transformations, it simply cannot be hardened by heating and quenching.
Low-Carbon Steels
Steels with very low carbon content (typically below 0.3%) do not have enough dissolved carbon to effectively trap and distort the crystal lattice during quenching. While they will form some martensite, the effect is minimal, and the resulting increase in hardness is not significant enough for most applications.
Austenitic Stainless Steels
This category, including the common 304 and 316 grades, is a prime example. Their high nickel and chromium content stabilizes the soft and ductile austenite (FCC) phase, even at room temperature. Because they do not transform out of the austenite phase upon cooling, quenching has no hardening effect.
Most Non-Ferrous Metals and Alloys
Metals like pure copper, pure aluminum, and many brasses or bronzes have a stable crystal structure that does not change with temperature. Without an allotropic phase transformation, the heating and quenching cycle simply heats the metal up and cools it back down, resulting in a softer, annealed state rather than a harder one.
Hardening Without Heat: The Alternatives
Just because a metal cannot be hardened by heat treatment does not mean it cannot be hardened at all. The primary alternative is mechanical.
Strain Hardening (Work Hardening)
This is the most common method for hardening the materials listed above. By physically deforming the metal at a temperature below its recrystallization point (i.e., "cold working"), we introduce dislocations and tangles into the crystal structure.
This internal chaos makes it more difficult for crystal planes to slip past one another, which manifests as an increase in hardness and strength. Processes like rolling, drawing, or bending all induce work hardening.
Solid-Solution Strengthening
This is a passive form of hardening achieved by alloying. Introducing atoms of a different size into the metal's crystal lattice creates localized strain and makes it harder for dislocations to move. This is why an alloy like brass (copper and zinc) is inherently harder than pure copper.
How to Apply This to Your Project
Your choice of material and hardening method depends entirely on the required final properties of the component.
- If your primary focus is extreme core hardness and wear resistance (e.g., cutting tools, dies, springs): You must select a hardenable steel with sufficient carbon content (typically >0.4%) and utilize a proper heat treatment and quench cycle.
- If your primary focus is corrosion resistance and ductility (e.g., food-grade tanks, marine hardware): An austenitic stainless steel (like 304) is ideal. If you need it to be harder, you must specify a work-hardened condition (e.g., "1/4 hard").
- If your primary focus is a tough, ductile core with a very hard surface (e.g., gears, shafts): A low-carbon steel is the perfect choice. It cannot be through-hardened, but its surface can be case-hardened via processes like carburizing or nitriding.
Understanding the fundamental "why" behind hardenability allows you to select the right material and process from the start, avoiding costly and ineffective treatments.
Summary Table:
| Metal Category | Key Examples | Reason for Non-Hardenability |
|---|---|---|
| Low-Carbon Steels | <0.3% Carbon Steels | Insufficient carbon for martensite formation |
| Austenitic Stainless Steels | 304, 316 | Stable FCC austenite phase at all temperatures |
| Pure Non-Ferrous Metals | Pure Copper, Pure Aluminum | No allotropic phase transformation |
Struggling to select the right metal for your application? KINTEK specializes in lab equipment and consumables for material testing and heat treatment processes. Our experts can help you choose the correct materials and methods to achieve your desired hardness and performance. Contact us today to optimize your project's success!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the conditions for tempering? Master the Heat Treatment Process for Stronger Steel
- How is a high-temperature heating furnace used to evaluate the thermal shock resistance of refractory materials?
- What is the physics of sintering? A Guide to Atomic Diffusion and Densification
- Why is a high-performance vacuum pump system critical for vacuum gasification? Unlock Efficiency and Purity
- Is brazing environmentally friendly? A Guide to Sustainable, Low-Impact Joining
- What is the function of a precision isothermal heating furnace in inducing secondary phase precipitation? Optimize Microstructures
- What are the outcomes of heat treatment? Tailor Material Properties for Superior Performance
- What is the temperature range required for pyrolysis? A Guide to Optimizing Biochar, Bio-Oil, and Syngas



















