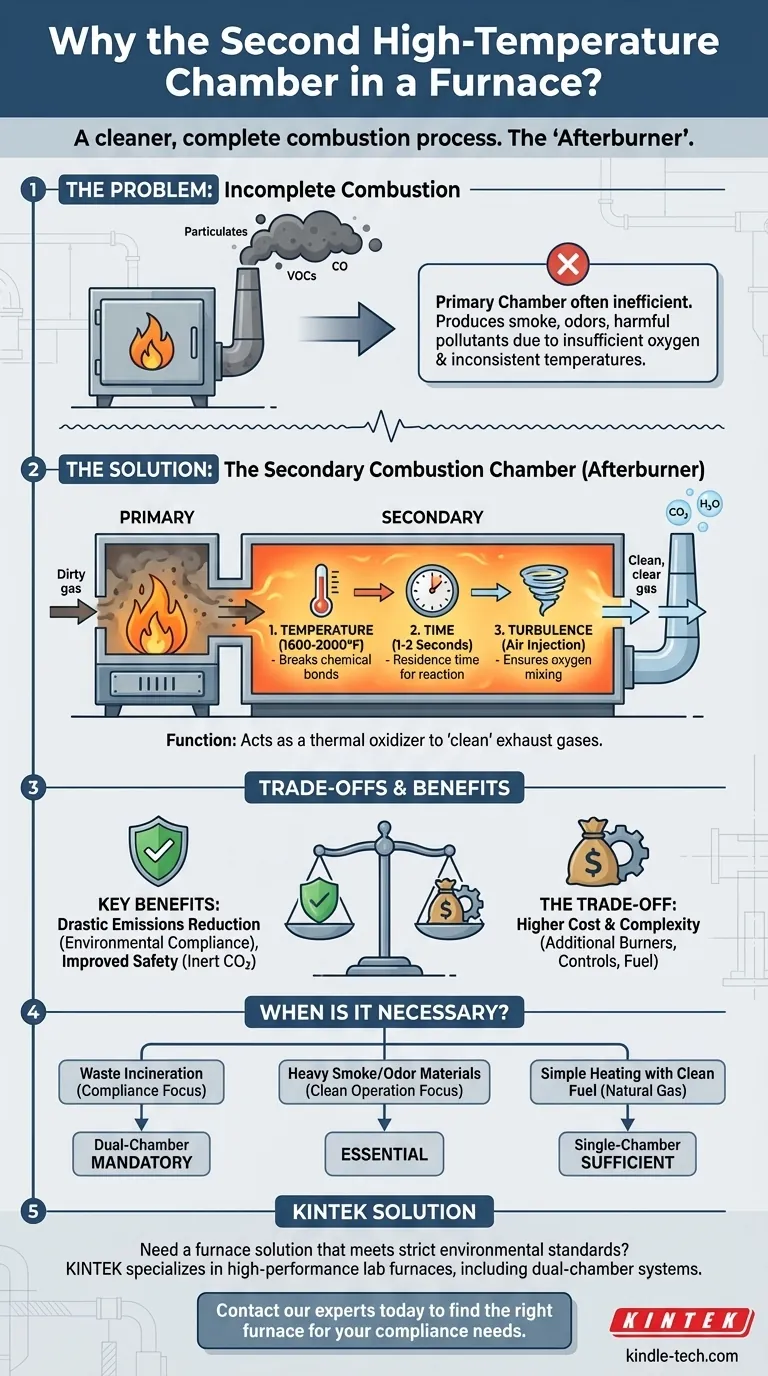
The second high-temperature chamber in a furnace is an afterburner. Its specific purpose is to receive the hot, dirty exhaust gas from the primary combustion chamber and destroy any remaining smoke, odor, and harmful pollutants. This is achieved by subjecting the gas to extremely high temperatures for a specific duration, ensuring a complete and clean combustion process before venting to the atmosphere.
The core problem is that burning material in a single chamber is often inefficient and dirty, creating pollutants. The second chamber acts as a sophisticated pollution control device, ensuring that what leaves the smokestack is primarily just carbon dioxide and water vapor.

The Problem: Incomplete Combustion in the Primary Chamber
What the Primary Chamber Does
The first, or primary, chamber is where the main work of burning the solid material (such as waste, wood, or other fuel) takes place. Its goal is to apply heat and break down the material through combustion.
Why Combustion Is Often Incomplete
Perfect combustion is difficult to achieve in the primary chamber. Factors like insufficient oxygen, inconsistent temperatures, and the complex nature of the material being burned lead to unwanted byproducts.
These byproducts include particulates (visible as smoke or soot), carbon monoxide (CO), and various volatile organic compounds (VOCs), which are often responsible for noxious odors. Releasing these directly into the atmosphere is polluting and often illegal.
The Solution: The Secondary Combustion Chamber
Its Core Function: An Afterburner
The second chamber functions as a thermal oxidizer, more commonly known as an afterburner. It doesn't burn solid material; its only job is to "clean" the exhaust gases produced by the primary chamber.
Mastering the "Three T's" of Destruction
To effectively destroy the pollutants, the secondary chamber is engineered to maintain three critical conditions, often called the "Three T's" of complete combustion.
1. Temperature
The secondary chamber is held at a consistently high temperature, often between 1,600°F and 2,000°F (870°C to 1100°C). This extreme heat is necessary to break the chemical bonds of harmful compounds like carbon monoxide and VOCs.
2. Time
The hot gases must remain in the secondary chamber for a specific period, known as residence time. A typical requirement is for the gases to be held at peak temperature for 1 to 2 seconds, providing enough time for the destructive chemical reactions to fully complete.
3. Turbulence
To ensure every pollutant molecule is destroyed, it must come into contact with oxygen at high temperature. Air is actively injected into the secondary chamber to create turbulence, promoting thorough mixing of the gases and oxygen.
Understanding the Trade-offs
Key Benefit: Drastic Emissions Reduction
The primary advantage is environmental compliance. A properly operating dual-chamber furnace can eliminate nearly all visible smoke, odors, and harmful emissions. This is essential for meeting strict air quality regulations set by agencies like the EPA, particularly in applications like waste incineration or cremation.
Key Benefit: Improved Safety
By converting flammable gases like carbon monoxide and VOCs into inert CO2, the system becomes safer. It prevents the release of potentially hazardous and combustible gases from the exhaust stack.
The Trade-off: Higher Cost and Complexity
Dual-chamber systems are more complex and expensive. They require additional burners, fuel, insulation, and sophisticated control systems to maintain the precise conditions in the secondary chamber. This results in higher initial capital costs and ongoing operational fuel expenses compared to a single-chamber design.
When is a Dual-Chamber Furnace Necessary?
Choosing the right furnace design depends entirely on the process requirements and regulatory obligations.
- If your primary focus is environmental compliance for waste incineration: A dual-chamber system is almost always mandatory to meet modern emissions standards.
- If your primary focus is processing materials that produce heavy smoke or odors: The secondary chamber is essential for clean operation and to be a good neighbor.
- If your primary focus is simple heating using a clean-burning fuel like natural gas: A single-chamber furnace is typically sufficient, as the fuel produces very few pollutants that would require a second chamber.
Ultimately, the inclusion of a second chamber transforms a simple furnace into a comprehensive processing system designed for clean and complete combustion.
Summary Table:
| Chamber | Primary Function | Key Outcome |
|---|---|---|
| Primary Chamber | Burns solid material (fuel, waste) | Produces heat and exhaust gases |
| Secondary Chamber (Afterburner) | Destroys pollutants in exhaust gas | Ensures clean emissions (CO₂, H₂O) |
Need a furnace solution that meets strict environmental standards?
KINTEK specializes in high-performance lab furnaces, including dual-chamber systems designed for complete, clean combustion. Our equipment ensures you can process materials efficiently while adhering to the strictest air quality regulations.
Contact our experts today to find the right furnace for your laboratory's specific needs and compliance requirements.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- How to design a pyrolysis reactor? Optimize for Biochar, Bio-oil, or Syngas Yield
- What are the benefits of vacuum drying? Achieve Gentle, Efficient Drying for Sensitive Materials
- What does it mean to sinter metals? A Guide to Solid-State Fusion for Strong, Complex Parts
- What is the medium of heat transfer in a vacuum? Harness Thermal Radiation for Precision Heating
- What materials can be vacuum cast? Polymer Prototypes vs. High-Performance Metal Parts
- What are the disadvantages of nitriding over carburizing? A Guide to Process Limitations
- What is the function of a high-frequency induction plasma reactor? Synthesis of Nano-scale Magnéli Phase Titanium Oxide
- Why is a high-vacuum diffusion pump system essential for MAX phase and Cu-Al melt experiments? Ensure Pure Interactions



















