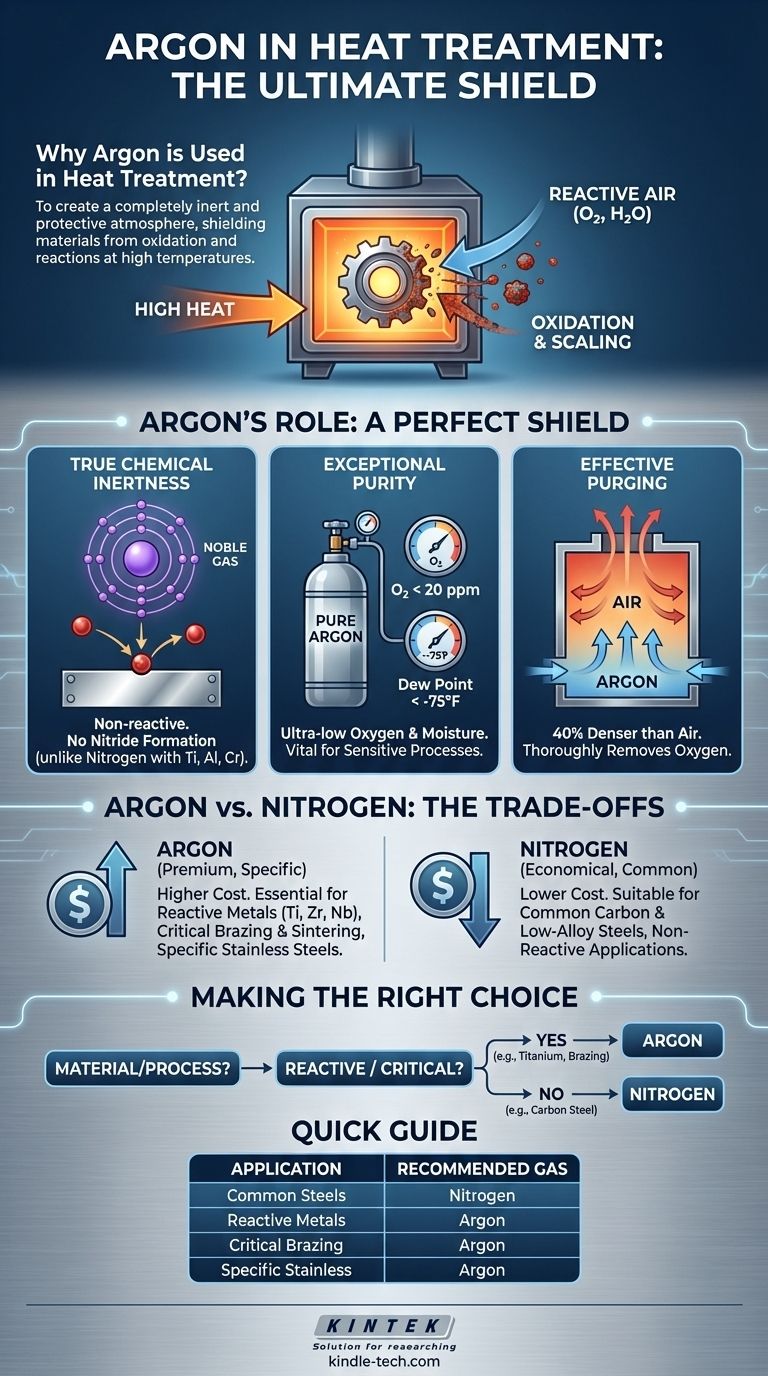
In heat treatment, argon is used to create a completely inert and protective atmosphere inside a furnace. At the high temperatures required for processes like annealing or brazing, metals become highly reactive with oxygen and moisture in the air. Argon, a noble gas, acts as a perfect shield, preventing these unwanted chemical reactions like oxidation and scaling, which would otherwise damage the component's surface and compromise its structural integrity.
The core reason to choose argon over more common protective gases like nitrogen is its absolute chemical inertness. While nitrogen is sufficient for many applications, argon is essential for highly sensitive or reactive materials where even the slightest surface reaction, such as nitride formation, is unacceptable.

The Fundamental Problem: Why an Atmosphere is Critical
Heat as a Catalyst for Unwanted Reactions
Heat treatment relies on precise temperature control to alter a metal's internal crystal structure, thereby changing its physical properties like hardness or ductility.
However, this same heat dramatically accelerates chemical reactions between the metal's surface and any reactive gases present.
The Threat of Oxygen and Moisture
Standard air is about 21% oxygen and contains variable amounts of water vapor. At heat-treating temperatures, both of these are aggressive oxidizing agents.
Contact with air will cause a metal part to form a layer of oxide scale on its surface. This damages the surface finish, alters dimensions, and can negatively impact the material's fatigue life and corrosion resistance.
The Goal: An Unchanged Surface
The objective of a protective atmosphere is to allow the thermal cycle to occur without altering the surface chemistry of the part. The component should exit the furnace with the same clean, bright surface it had going in.
Argon's Role as a Protective Shield
True Chemical Inertness
Argon's primary advantage is that it is a noble gas. Its outer electron shell is full, making it chemically non-reactive with all other elements under furnace conditions. It provides a truly inert environment.
This is a critical distinction from nitrogen, which, while largely unreactive, can form nitrides with certain elements like titanium, aluminum, and the chromium found in some stainless steels. This nitride formation can alter the material's properties in undesirable ways.
Exceptional Purity and Low Dew Point
Industrial-grade argon is delivered at extremely high purity. Specifications of an oxygen content below 20 parts per million (ppm) and a dew point below -75°F (-59°C) are common.
This means the gas is exceptionally free of the two main culprits—oxygen and water vapor. This high level of purity ensures that no oxidation can occur, which is vital for sensitive processes like brazing, where any oxide layer can prevent the filler metal from properly wetting and bonding to the base materials.
Effective Furnace Purging
Argon gas is approximately 40% denser than air and about 43% denser than nitrogen. This property can be used to effectively purge a furnace of atmospheric air.
When introduced at the bottom of a furnace chamber, the heavier argon displaces the lighter air, pushing it out through top vents. This method ensures a thorough and efficient removal of oxygen before the heating cycle begins.
Understanding the Trade-offs: Argon vs. Nitrogen
The Primary Factor: Cost
The most significant disadvantage of argon is its cost. It is produced by fractional distillation of liquid air, a process that makes it considerably more expensive than nitrogen, which is the most abundant gas in the atmosphere.
Because of this cost difference, argon is only used when technically necessary.
When Nitrogen is "Good Enough"
For a vast range of heat-treating applications, particularly for common carbon and low-alloy steels, nitrogen provides a perfectly suitable protective atmosphere. It effectively prevents oxidation and is the most economical choice.
In these cases, the metals being treated are not susceptible to nitride formation, so the added protection (and cost) of argon is unnecessary.
When Argon is Non-Negotiable
Argon becomes the required choice for specific materials and processes where nitrogen poses a risk.
This includes heat treating reactive metals like titanium, zirconium, and niobium. It is also critical for certain stainless steels and nickel alloys where chromium nitride formation would deplete the surface of chromium, reducing its corrosion resistance. Finally, high-purity argon is often specified for critical brazing and sintering operations where a perfectly clean surface is paramount for success.
Making the Right Choice for Your Application
Selecting the right protective atmosphere is a balance between process requirements and cost. Your decision must be based on the material being treated and the intolerance for any surface reaction.
- If your primary focus is cost-efficiency for common carbon or low-alloy steels: Nitrogen is almost always the correct and most economical choice for preventing general oxidation.
- If you are treating reactive metals like titanium or specific stainless steels: Argon is essential to prevent the formation of unwanted nitrides that would compromise the material's inherent properties.
- If your process involves critical brazing or powder metal sintering: The superior purity and complete inertness of argon are required to ensure a flawless surface for proper bonding and densification.
Ultimately, choosing the right gas is a critical engineering decision that directly protects the integrity and value of your finished component.
Summary Table:
| Application Scenario | Recommended Atmosphere | Key Reason |
|---|---|---|
| Common Carbon/Low-Alloy Steels | Nitrogen | Cost-effective oxidation prevention |
| Reactive Metals (Titanium, Zirconium) | Argon | Prevents nitride formation, ensures chemical inertness |
| Critical Brazing & Sintering | Argon | High purity prevents surface contamination for proper bonding |
| Specific Stainless Steels & Nickel Alloys | Argon | Avoids chromium depletion from nitride formation |
Protect your most sensitive materials and critical processes with the right atmosphere solution.
Choosing between argon and nitrogen is a crucial decision that directly impacts your component's quality and performance. The experts at KINTEK specialize in providing laboratory equipment and consumables, including atmosphere control solutions for heat treatment. We can help you determine the optimal protective atmosphere for your specific materials and applications, ensuring flawless results and protecting your investment.
Contact our experts today for a personalized consultation on your heat treatment atmosphere needs.
Visual Guide
Related Products
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What is the use of hydrogen in furnace? A Key to Oxygen-Free High-Temperature Processing
- What are the primary advantages and disadvantages of using an oxygen probe? Optimize Your Atmosphere Control Strategy
- How to create an inert atmosphere in a furnace? Master the Vacuum-Purge Method for Oxidation-Free Results
- Why hydrogen is used in furnace? Unlock Faster Heating & Purer Metal Surfaces
- What is the dew point of a furnace atmosphere? Master Heat Treatment Quality and Control
- What is the function of a high vacuum atmosphere furnace in validating hydrogen diffusion models? Ensure Pure Data.
- What causes oxidation in heat treatment? Control Your Furnace Atmosphere to Prevent Scale & Decarburization
- What function does a high-temperature atmosphere furnace serve in catalyst activation? Boost Platinum Performance



















