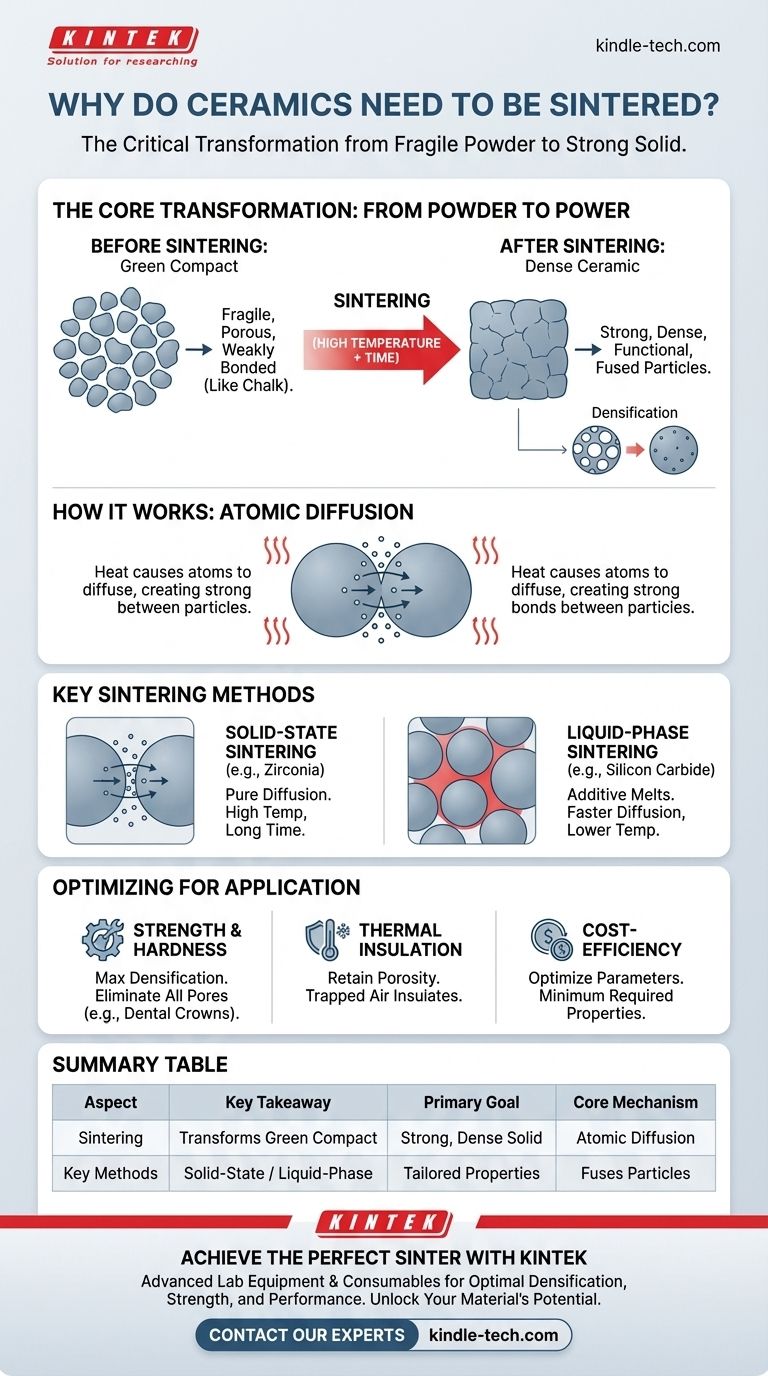
In essence, sintering is the critical manufacturing step that transforms a fragile, compacted ceramic powder into a strong, dense, and functional solid. Without this high-temperature process, the individual ceramic particles would remain weakly bonded, resulting in a material unable to withstand mechanical stress or serve any practical purpose. Sintering fundamentally changes the microstructure to create a robust and stable final product.
A ceramic object before sintering, known as a "green compact," is merely a collection of pressed-together particles with significant empty space. Sintering is the engineered process of using heat to fuse these particles, drastically reducing porosity and creating the strength and durability required for real-world applications.

From Powder to Power: The Core Transformation
Sintering is not simply baking; it is a complex process of mass transport on an atomic scale. Understanding this transformation is key to appreciating why it is indispensable for all advanced ceramics.
The "Green" State: A Fragile Beginning
Before sintering, ceramic powders are shaped into the desired form using methods like pressing or casting. This initial object, called a green compact, has mechanical integrity but is highly porous and fragile, similar in consistency to a piece of chalk.
The particles are only held together by weak physical forces, and the object has none of the desired properties like hardness or thermal stability.
The Role of Heat and Diffusion
When heated to a high temperature—typically below the material's melting point—the atoms at the contact points between particles become highly agitated. This thermal energy allows them to diffuse, or move, across the boundaries of adjacent particles.
This atomic movement effectively builds "bridges" between the particles, slowly fusing them together into a single, solid mass known as a polycrystalline material.
Achieving Densification
As the particles fuse, the empty spaces, or pores, between them shrink and are gradually eliminated. This process is called densification.
A denser ceramic is a stronger ceramic because pores act as stress concentration points where fractures can begin. By removing these weak points, sintering dramatically increases the material's mechanical strength, hardness, and fracture resistance.
Key Sintering Mechanisms
The exact method of sintering is chosen based on the ceramic material and the desired final properties. The two primary mechanisms are solid-state and liquid-phase sintering.
Solid-State Sintering
Used for materials like zirconia and alumina, this method relies purely on atomic diffusion through the solid particles. It requires very high temperatures and often longer processing times because moving atoms through a solid structure is a slow process.
Liquid-Phase Sintering
For materials that are difficult to densify, such as silicon carbide, a small amount of an additive is mixed with the ceramic powder. At sintering temperatures, this additive melts and forms a liquid phase.
This liquid wets the ceramic particles and pulls them together through capillary forces, much like water pulls grains of sand together. It provides a faster path for diffusion, allowing for lower sintering temperatures and shorter times.
Understanding the Trade-offs and Controls
Sintering is not a one-size-fits-all process. The parameters are carefully engineered to achieve a specific outcome, and there are always trade-offs to consider.
The Porosity Problem
While the goal is often to eliminate porosity, any remaining pores will degrade the material's performance. For a high-stress application like a dental ceramic crown, which must withstand chewing forces, even a small amount of porosity can lead to catastrophic failure.
The Temperature and Time Equation
Higher temperatures and longer sintering times generally lead to greater densification. However, they also increase energy costs and can cause undesirable grain growth, which can sometimes make the material more brittle. The goal is to find the optimal balance for the specific application.
The Impact of Pressure
Applying external pressure during the heating cycle, a technique known as hot pressing, can significantly enhance densification. The pressure physically forces the particles together, helping to close pores more effectively and allowing for the use of lower temperatures or shorter times.
How Sintering Achieves Specific Goals
The choice of sintering parameters is driven entirely by the intended application of the final ceramic part. Your approach should be tailored to the primary performance requirement.
- If your primary focus is maximum strength and hardness: Your goal is to achieve near-total densification by using high temperatures, long durations, or pressure-assisted sintering to eliminate virtually all porosity.
- If your primary focus is thermal insulation: You might intentionally control sintering to retain a specific level of porosity, as the trapped air in the pores acts as an excellent barrier to heat transfer.
- If your primary focus is cost-effective production: You will optimize sintering parameters (temperature, time, additives) to achieve the minimum required properties for the application, such as for a ceramic tile, in the shortest possible time.
Ultimately, sintering is the essential engineering step that unlocks the inherent potential of ceramic materials, transforming them from raw powder into highly functional components.
Summary Table:
| Sintering Aspect | Key Takeaway |
|---|---|
| Primary Goal | Transforms weak green compact into a strong, dense solid. |
| Core Mechanism | Atomic diffusion fuses particles, eliminating pores. |
| Key Methods | Solid-state sintering (e.g., zirconia) or liquid-phase sintering (e.g., silicon carbide). |
| Application Focus | Tailor sintering parameters for strength, insulation, or cost-efficiency. |
Ready to achieve the perfect sinter for your ceramic components?
At KINTEK, we specialize in providing advanced lab equipment and consumables tailored to your sintering needs. Whether you're developing high-strength dental crowns, thermal insulators, or cost-effective industrial ceramics, our expertise ensures optimal densification, strength, and performance.
Let us help you unlock the full potential of your materials. Contact our experts today to discuss your specific requirements and discover how KINTEK can enhance your sintering process.
Visual Guide
Related Products
- 1200℃ Muffle Furnace Oven for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What experimental design method was utilized in the study of grinding composite ceramic? Optimizing Process Efficiency
- How high temperature can ceramic withstand? A Guide to Extreme Heat Performance
- What are the different types of ceramic styles? A Guide to Earthenware, Stoneware, Porcelain & Bone China
- What temperature does clay sinter? Mastering the Range for Perfect Ceramic Results
- What is the purpose of using ceramic fiber insulation in molten salt pipelines? Ensure Fluidity and Energy Efficiency
- What is the strength of dental ceramics? Mastering the Compressive vs. Tensile Force Balance
- At what temperature does ceramic melt? A Guide to Ceramic Heat Resistance
- What is another name for dental ceramic? Discover the Porcelain & Modern Material Options



















